Fig. 6.1
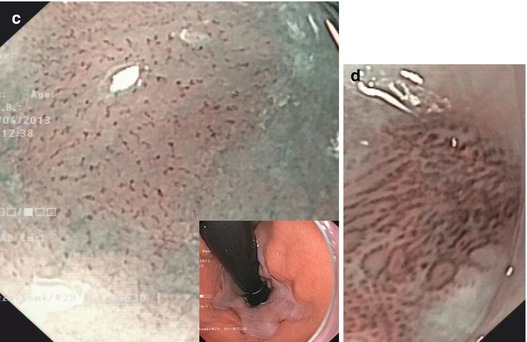
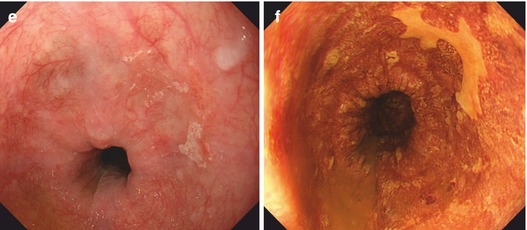
Normal squamous epithelial esophagus. (a) Standard WLI with dendritic submucosal vascular pattern (and very faint IPCLs), (b) squamous epithelial esophagus with regular straight IPCL pattern (loop A JES; CP I, Inoue), M-NBI (60×) (c) IPCL pattern normal loop type A, JES (type II, Inoue, with elongation of IPCL). M-NBI 40×. Mild post-esophagitic changes of SC mucosa (left, lower esophagus) in 70-year-old woman with axial hernia and chronic GERD on treatment with omeprazol (insert: cardia in retroflex view, WLI). (d) IPCL pattern normal loop type A, JES (types II and III, Inoue), with elongation, some variation of shape. M-NBI 60×, same patient as in (c) (e) Early squamous cell cancer type 0-IIa + b on WLI (left). (f) Lugol-CE (right) shows unstained SCC and mild “pink color sign”
Signs of mucosal neoplasias on standard WLI endoscopy are areas with:
Slightly reddish appearance (as compared to regular mucosa)
Or tiny whitish coating (keratinizing type SCC)
Uneven, velvety to granular appearing surface structure
Disappearance of normal mucosal light reflex
Disappearance of dendritic vascular network of submucosal veins
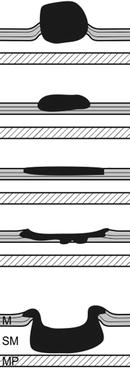
Macroscopic types of early SC neoplasias. Earliest stages (HGIN, SCC pT1a) confined to EP (m1) or LPM (m2) exhibit types 0-IIb by macroscopic classification of neoplasias (Table 4.4). The likelihood of sm2 invasion is about 30 % in slightly depressed neoplasias (0-IIc) and increases to about 50 % with elevated type (0-IIa or 0-IIa + IIc) – paralleled by diagnostic alterations in IPCL pattern. Protuberant (0-Is) or ulcerated SCC (0-III) usually are deeply sm invasive. Table 6.1 presents the prevalence and risk of sm invasiveness of early SCC types diagnosed before introduction of magnifying NBI analysis [3], a fact that explains why the relative prevalence of 0-IIb and 0-IIa types was low among early esophageal SCC.
Table 6.1
Esophageal early squamous cell cancer (ESCC)
Type | Prevalence (% of ESCCa) | Depth of tumor invasion (percent of macroscopic type) | |||
|---|---|---|---|---|---|
M1 & M2 | M3 & sm1 | ≥sm2 | |||
 | 0-I (s, p) | 14 | 4 | 17 | 79 |
0-IIa | 16 | 20 | 31 | 49 | |
0-IIb | 12 | 69 | 16 | 15 | |
0-IIc | 38 | 36 | 35 | 29 | |
0-IIa + IIc | 8 | 10 | 37 | 53 | |
0-III | 4 | 3 | 13 | 83 | |
Chromoendoscopy with 0.75–1 % iodine solution is useful for detection and analysis of lateral margins of superficial squamous cell cancer:
However, the problem is inflammation caused by Lugol. Sometimes the patient feels heartburn. And rarely, it causes shock.
6.4 Endoscopic Diagnosis of Mucosal Lesions Based on Magnifying NBI Endoscopy: Basic Microvascular Patterns (MVP)
Normal epithelium in squamous esophagus shows regular pattern of intrapapillary capillary loops (IPCL) which is well enhanced on magnifying NBI observation. Magnifying NBI (>60-fold) reveals a striking microvessel pattern (MVP) that consists of a longitudinal array of fine parallel straight IPCLs in the lamina propria mucosae (MVP type I, Inoue) and a branching pattern of thicker arterioles and oblique venules in the submucosa (Fig. 6.1a, b). Alterations of mucosal MVP are the key to accurate endoscopic diagnosis of mucosal lesions (Table 6.2). MVP types are defined by alterations in four IPCL characteristics – length (elongation), tortuosity, caliber (thickness), and shape (distortion of loop) – and reflect changes in anatomy of epithelial papillae, which are destroyed by vertical tumor invasion and neoangiogenesis.
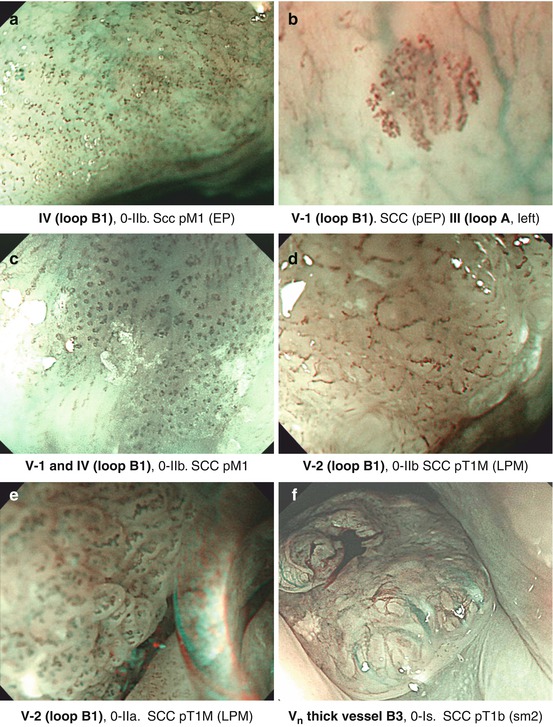
Table 6.2
Microvascular pattern (MVP) of squamous cell-lined esophagus (on NBI image)
Inoue classification (modified)a | Histopathology | Japan Esophageal Soc. (JES). Classificationb | |||
|---|---|---|---|---|---|
MVP type | IPCL | Invasion | LN+ | IPCL pattern | MVP type |
I |  | Nonneoplastic | Normal IPCL pattern | A | |
II |  | ||||
III |  c c | ||||
IV |  c c | EP | 0–1 % | Abnormal loop pattern | B1 |
V-1 |  c c | m1 | 2 % (1–9 %) | ||
V-2 |  c c | m2 | |||
V-3 |  c c | m3 (mm)/sm1 | 10/20 % | Non-loop pattern | B2 |
Vn |  c c | sm2 (massive invasion) | ~50 % | Very thick (tumor) vessel | B3 |

Fig. 6.2
(a–f) Microvascular pattern types (compare Table 6.2) of squamous cell neoplasias, magnifying NBI (60–100-fold), and corresponding histology obtained after ESD
Nonneoplastic lesions present variants of normal pattern: IPCL type II (IPCL elongation, slightly Lugol staining) (Fig. 6.1c) and IPCL type III (prominent elongation, discrete tortuosity of some IPCL; Lugol voiding) which are typical of mild acute or severe chronic esophagitis (Fig. 6.1d).
Neoplasias, when flat, appear as slightly reddish or (keratinizing) whitish areas that remain unstained on Lugol-CE and often show “pink-color sign” few minutes after Lugol spraying (Fig. 6.1e, f). They are readily apparent on NBI as brownish areas that display on magnifying NBI augmented and irregular MVP and loss of normal dendritic sm vascular pattern (Fig. 6.2a–f). Inoue’s MVP classification of squamous esophagus has been most widely applied [7, 10, 11] but has now been simplified by consensus of the Japan Esophageal Society to the principal IPCL patterns A, B1, B2, and B3 (Table 6.2) (Fig. 6.2a–f; compare Fig. 6.5h). Neoplastic changes in caliber and shape of IPCL, with preserved IPCL loop configuration, progressive curling and slight dilatation of IPCL tops, and elongation of IPCL, are typical for dysplasia (LGIN or HGIN) or intramucosal carcinoma (M1, M2) and classified as MVP abnormal loop B 1 , JES (type IV, V–1, V–2, Inoue).
SCC extending to the muscularis mucosae (M3) or minimally into submucosa (sm1) typically show some vanishing IPCL – due to destruction of epithelial papillae – and/or marked elongation of IPCL and connection of adjacent IPCL yielding MVP type V–3 (JES non–loop type B 2 ). Distinctly nonstructured, disrupted MVP type V N (JES thick vessel type B 3 ) exhibits all four abnormalities including thick abnormal tumor vessels and is characteristic for SC carcinoma with deep sm invasion (Fig. 6.2f) [7, 10, 12].
Note
In squamous cell-lined esophagus, NBI reveals changes in MVP of IPCL which permit to distinguish with high accuracy between [10, 13, 14]:
Nonneoplastic (MVP I, II/A) vs. neoplastic lesions (MVP IV–Vn/B)
Intramucosal HGIN/sm-microinvasive carcinoma (MVP IV–V3/B1 (B2) vs. carcinoma with deep submucosal invasion (MVP Vn/B3)
6.5 Endoscopic Diagnosis of Nonneoplastic and Neoplastic Lesions
Reddish flat lesions in squamous cell-lined esophagus cover a variety of inflammatory and nonneoplastic lesions as well as neoplastic lesions. For classification of macroscopic type of lesions, compare Fig. 4.2a. Most are esophagitic lesions (erosions, flat ulcerations, inflammatory hyperplasia) of different etiology (e.g., Fig. 6.3a) [15]. All these show inflammatory IPCL pattern type II or III (Inoue), i.e., normal loop type A, JES. Mechanical damage may cause IPCL hematomas (Fig. 6.3c–e). Severe ischemic damage may present livide–dark areas, the so-called black esophagus (with IPCL type A). This differs from pigmented melanosis (Fig. 6.3f) – a sign of past toxic exposure with risk of squamous cancer [1, 16]. Similar, pigmented submucosal lesions may show pigmented nevi or malignant melanoma on biopsy.
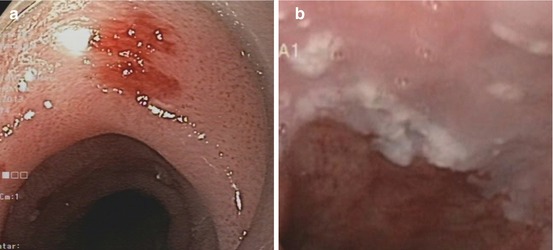
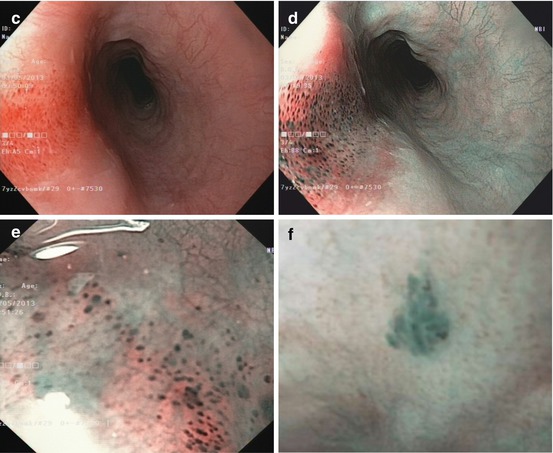
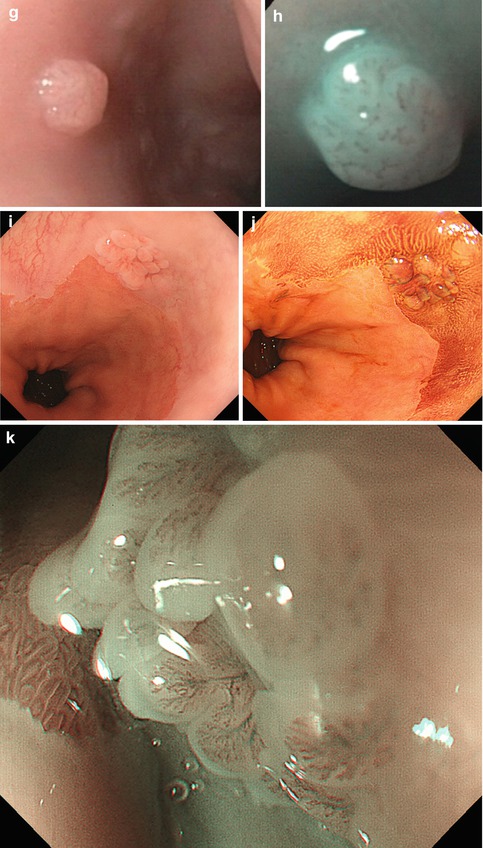
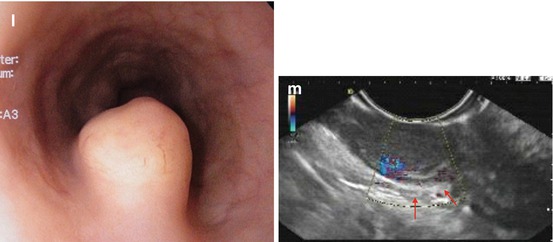




Fig. 6.3




(a) Acute inflammatory lesion in lower esophagus caused by pancreatobiliary reflux after removal of enteric drainage tube. Patient with Billroth-II partial gastrectomy. (b) Candida esophagitis at GE junction, white fungal plaques, WLI (Note: Epithelial lesion with white fungus plaque, without neovascularization) ((c–e) Lesion 0-IIa (14–16 cm p.i., anterior wall). (c) Squamous epithelial damage (IPCL microhematomas) caused by mechanical shear stress within upper esophageal sphincter area WLI, (d) NBI, (e) M-NBI 60-fold. Case: 59-year-old healthy woman with cricopharyngeal dysphagia. Histology: regular epithelial papillas and squamous epithelial layer with loss of superficial epithelial cell layers. (f) Pigmented melanosis in normal squamous mid-esophagus NBI (20×) in 65-year-old healthy woman (g–k) Papillomas in squamous esophagus. (g) WLI: whitish, 0-IIa-like; (h) M-NBI (40×): with IPCL pattern II; (i) WLI: papilloma in squamous cell-lined esophagus next to hiatal hernia; (j) Lugol stain: mildly Lugol staining to Lugol-voiding lesion (WLI, 4-fold zoom), (k) sea anemone-like appearance (IPCL loop type A, JES) by M-NBI 100-fold ((l) Pale SMT (size >10 mm) covered with normal squamous epithelium in mid-esophagus, WLI. (m) EUS (7.5 MHz) shows an hypoechoic lesion contiguous with sm layer (↑), FNP reveals S-100 expressing granular cell tumor (Abrikossof) (Courtesy by G. Kleber (resected en bloc R0 with ESD, nonmalignant according to Ki-67 index))
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








