Fig. 8.1
The anterograde Spirus EndoEase Discovery SB overtube utilized primarily for anterograde deep enteroscopy. The same overtube can be used for retrograde procedures but only with a small bowel enteroscope. Reprinted with permission of Spirus Medical LLC
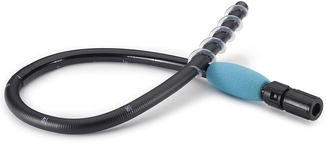
Fig. 8.2
The retrograde Spirus EndoEase Vista overtube is shorter and wider than its anterograde counterpart. It can be used with all small bowel enteroscopes as well as some pediatric colonoscopes with diameter <11 mm. Reprinted with permission of Spirus Medical LLC
Two operators are usually required to perform the spiral enteroscopy technique given the fact that both the overtube and the instrument have to be manipulated during the procedure. The overtube is installed on the enteroscope using an interlocking device, which can switch between a longitudinal (advance-withdrawal) and rotational axis of freedom for the scope within the overtube. The procedure can be performed with moderate sedation, monitored anesthesia care (deep sedation), or with general anesthesia depending on patient, indication, and operator variables. If general anesthesia is used for anterograde procedures, it is advisable to deflate the endotracheal balloon while the spiral is advanced through the upper esophagus to avoid trauma. Infrequently, in patients with significant cervical spine disease or cervical osteophytes, the overtube cannot be advanced past the upper esophagus and an alternative enteroscopy method has to be employed [2]. Once past the upper esophageal sphincter, the fixed overtube–enteroscope unit is carefully advanced through steady rotation through the stomach into the duodenum, keeping in mind the possibility of occult strictures. Nonobstructive esophageal Schatzki’s rings are usually inconsequential, but strictures less than 15 mm in diameter should be traversed with caution. Once the overtube engages the pylorus and duodenum, the scope–overtube unit usually advances fairly easily with steady clockwise rotation into the small bowel. When the entire effective part of the overtube has been inserted and rotational advancement stops or when the operator encounters unusual rotational resistance, the unit can be unlocked again and the scope advanced independently in the small bowel to the maximal point of insertion or until pathology is found.
For the retrograde approach, the overtube serves primarily to “splint” the endoscope (usually an enteroscope) during insertion into the colon and avoid looping (Fig. 8.3). The retrograde spiral overtube can rarely be engaged through the ileocecal valve. Instead, in a relatively straight configuration and under favorable valve orientation, the enteroscope itself can be advanced relatively easily in the ileum (Video 8.1). In a small study, the terminal ileum was intubated in 100 % of patients and the depth of insertion past the ileocecal valve was estimated at 100 cm (range 50–150 cm) [3]. Controlled visualization and endoscopic therapy take place during withdrawal, which is essentially the reverse of the process described previously (i.e., counter-clockwise rotation of the overtube with the scope either in “locked” or “free” position). In order to increase the traction of the overtube on the small bowel, only the minimum amount of gas (air or CO2) is insufflated during advancement. A more detailed description of the procedure is available [1].


Fig. 8.3
The retrograde Spirus Vista overtube assembled on a 250 cm enteroscope. Note that the distal 20 cm of the scope are extending outside of the overtube during insertion to allow mobility of the scope and avoid excessive tension on the bowel wall. Some endoscopists prefer to have the overtube withdrawn all the way to the scope hub when they introduce the instrument through the rectum. After the scope is introduced for at least 60–70 cm, the colon loops are straightened and the overtube is advanced through the anus by gentle rotation while the colon lumen is kept in the field of view of the scope. Reprinted with permission of Spirus Medical LLC
Spiral enteroscopy has the same indications as other device-assisted enteroscopies. In addition, it has the advantage of being a latex-free device and thus it can be utilized safely in patients with known latex allergy. Although no definite contraindications have been formulated, patients with severe luminal strictures (including surgical anastomoses, Crohn’s disease, radiation enteropathy) esophageal varices, pregnancy, or severe coagulopathy have been excluded from most studies [2, 4].
Technical Success
The technical success rate, defined as the ability of the instrument to advance past the proximal jejunum in patients with normal anatomy, is approximately 95 % [2]. The most common reasons for failure are luminal strictures, abnormal or unusual anatomy (J-shaped stomach or narrow duodenal sweep) and anesthesia instability [2]. The maximal depth of insertion using spiral enteroscopy is on average 200–250 cm post-pyloric (range 10–600) and roughly corresponds to an area between the distal jejunum and proximal ileum, although these measurements have not been adequately validated [2, 5–7]. No clear predictors of the depth of insertion have been identified, although this is an important aspect of these procedures [8]. The average time to reach the maximum depth of insertion is variable, but in general it is shorter than either single- or double-balloon enteroscopy [4, 9, 10]. Akerman et al. reported an average insertion time of 18.7 min and total procedure time of 29 min [5]. In a US multicenter trial, the maximal extent was reached in an average of 22.1 ± 11.5 min and the mean total procedure time was 34.4 ± 10.1 min for diagnostic enteroscopies and 11.4 min longer (range 0–73 min) for those involving therapeutic interventions [2]. However, the depth of insertion and the rate of complete or panenteroscopy achieved with spiral enteroscopy appears to be inferior to that of double-balloon enteroscopy. In a small study using a combined anterograde and retrograde approach, pan-enteroscopy was accomplished in only 8 % of patients using spiral enteroscopy versus 92 % with DBE [4]. Spiral enteroscopy can be used successfully to access bypassed areas of the intestine in patients with altered anatomy such as Roux-en-Y anastomoses. In a small retrospective study, there was no difference in the diagnostic and therapeutic yield of spiral enteroscopy and SBE when both were used as platforms to perform endoscopic retrograde cholangiopancreatography (ERCP) in patients with altered anatomy including gastric bypass [11].
The learning curve with spiral enteroscopy seems to be relatively short. A selected group of experienced gastroenterologists was able to acquire the skills for spiral enteroscopy with fewer than ten procedures in a dedicated training environment [12].
Diagnostic and Therapeutic Yield
The diagnostic and therapeutic yield of spiral enteroscopy in patients with suspected small bowel disorders is similar to other device-assisted deep enteroscopy techniques. Significant small bowel abnormalities are found in 33–75 % of symptomatic patients [2, 9, 10, 13]. Selecting patients via preliminary noninvasive studies such as capsule endoscopy increases the yield [2, 7, 9]. Diagnostic and therapeutic interventions can be performed in over 70 % of patients with positive findings [2, 9]. One potential advantage of spiral enteroscopy over other methods is that the endoscope can be withdrawn completely from the patient while maintaining the overtube in a stable position, thus allowing repetitive maneuvers such as piecemeal polypectomy or foreign body retrieval.
Comparison with Other Deep Enteroscopy Techniques
Several small studies compared the technical performance and diagnostic yield of spiral enteroscopy with double-balloon (DBE) or single-balloon enteroscopy (SBE) [4, 9, 14, 15]. The only randomized trial including 26 patients found that the depth of insertion and the ability to perform bidirectional panenteroscopy (combining oral and anal approaches) was significantly higher with DBE compared to spiral enteroscopy (92 % versus 8 %, p = 0.002) but at the expense of a longer procedure time. However, the diagnostic and therapeutic yields were similar [4]. In contrast, a multicenter larger prospective cohort study found no difference in insertion depth, procedure duration, diagnostic and therapeutic yield between the two techniques. Panenteroscopy was not attempted in this study [9]. In a retrospective single-center study, the average depth of maximal insertion was found to be higher with spiral enteroscopy than SBE (301 cm versus 222 cm, p < 0.001) but procedure duration and diagnostic yield were not significantly different, although there was a trend for longer procedure time with SBE [10]. A comparison of the three most popular deep enteroscopy modalities is provided in Table 8.1. Overall it appears that spiral enteroscopy is faster than either balloon-assisted procedure, but the depth of insertion is less than that of DBE and may be superior to that of SBE.
Table 8.1
Performance comparison of the three most popular deep enteroscopy techniques
Spiral enteroscopya
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|





