Fig. 5.1
The human spermatozoa, testis, and epididymis. To the left is a mature human spermatozoon showing the components that make up the head, midpiece and tail sections. To the right is a view of the human testis and the seminiferous tubules, as well as the epididymis, showing the corpus ( head) and caudal ( tail) sections. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2010–2013. All rights reserved.)
Each testis is composed of two distinct compartments: (1) the tubular compartment that contains the seminiferous tubules and (2) the intertubular compartment that lies between the seminiferous tubules and contains the interstitial tissue. Each of these compartments is anatomically separate but remains closely linked together. Within the seminiferous tubules are the spermatogonial germ and Sertoli cells. The Sertoli cells provide a hormonally active environment for the evolution of primitive germ cells into mature male gametes or spermatozoa .
The bulk (90 %) of the testicular volume is made up of the seminiferous tubules and the germ cells that lie within the invaginations of the Sertoli cells, which make up the germinal epithelium. The seminiferous tubules also consist of peritubular tissue or lamina propria [6]. The peritubular tissue contains myofibroblasts that cause peristaltic contractions of the seminiferous tubules. This movement helps to transport the developing, immotile germ cells to the rete testis [7]. The intertubular spaces within the lobules contain clusters of Leydig or interstitial cells that make up the endocrine portion of the testis. The interstitial tissue consists primarily of blood and lymph vessels, nerve and collagenous fibers, macrophages, and a variety of connective tissue cells. The spermatogenic process is dependent on intra- and extratesticular hormonal regulatory processes, the functions of the intertubular microvasculature, Leydig cells, and other cellular components in the interstitium (intertubular space) [8].
The testis is responsible for synthesizing (steroidogenesis) and secreting androgens (i.e., testosterone), which is directly interrelated to its second function, producing spermatozoa (spermatogenesis) . These functions are under hormonal control via the pituitary gonadotropins—luteinizing hormone (LH) , and follicle-stimulating hormone (FSH) .
Hormonal Control of Spermatogenesis (Extrinsic Influences)
The hormonal regulation of spermatogenesis is under the control of the hypothalamus–pituitary–gonadal (HPG) axis. This axis begins as the higher center sends signals to the hypothalamus, which acts as the integrating center. The hypothalamus releases gonadotropin releasing hormone (GnRH) in discrete pulses that peak every 1.5 h. GnRH acts on the anterior pituitary to stimulate gonadotropin production (LH and FSH). A continuous production of GnRH will cause gonadotrophin desensitization, which will diminish LH and FSH release. LH is released in a similar pulsatile pattern to that of GnRH while FSH release is influenced by inhibin. LH and FSH act on the testes to produce testosterone and inhibin, respectively. LH acts on the Leydig cells in the testes to stimulate testosterone production through the conversion of cholesterol. When testosterone levels accumulate, it exerts a negative feedback effect at the pituitary (short loop) to suppress the release of LH and at the hypothalamus (long loop), which ultimately suppresses GnRH production and thereby regulates testosterone levels. FSH acts on the Sertoli cells to stimulate inhibin and androgen-binding protein (ABP) secretion. Accumulating inhibin levels exert a negative feedback effect at the pituitary to suppress FSH release, thereby regulating inhibin levels.
FSH is required at the onset of puberty to initiate spermatogenesis as its action on Sertoli cells is necessary for germ cell maturation. Testosterone is essential for maintaining the spermatogenic process. Its actions are facilitated by the Sertoli cells. Spermatocytes have ABP receptors but not androgen receptors whereas the Sertoli cells have androgen receptors. The binding of ABP to testosterone may assist testosterone movement toward the lumen of the seminiferous tubule onwards to the epididymis. FSH also induces the conversion of testosterone to 5α-dihydrotestosterone (5α-DHT) and 17β-estradiol. 5α-DHT is more active than testosterone and along with 17β-estradiol, is involved in the development and function of the penis, scrotum, accessory sex glands, secondary sex characteristics, libido and potency .
Leydig Cells
Leydig cells are embedded in groups that surround the connective tissue between seminiferous tubules in the testicle. These endocrine cells are the principal source of testosterone, the production of which is stimulated by LH (Table 5.1). In adults, testosterone in circulation is kept within the physiological range of 300–1200 ng/dL while intratesticular levels of testosterone are far higher. In the testes, testosterone levels are highest at the basement membrane of the seminiferous tubules.
Table 5.1
Functions of the Leydig and Sertoli cells
Functions of the Leydig cells | Functions of the Sertoli cells |
|---|---|
Initiation and maintenance of spermatogenesis | Maintains the integrity of seminiferous tubules epithelium |
Activation of the hypothalamus–pituitary–gonadal axis | Secretion of hormones—inhibin and androgen-binding protein (ABP) |
Production of testosterone—manifestation of male secondary sex characteristics | Secretes tubular fluid into the tubular lumen for transport of sperm within the duct |
Differentiation of male genital organs | Delivery of nutrients to germ cells |
Masculinization of the brain and sexual behavior | Steroidogenesis and steroid metabolism |
– | Aids in process of phagocytosis and elimination of cytoplasm |
– | Regulates the spermatogenic cycle |
– | Acts as a hormonal target for LH, FSH, and testosterone |
Testosterone
Testosterone, the major male androgen in circulation and in the Leydig cells, is responsible for primary and secondary sex characteristics. It is synthesized from cholesterol in the Leydig cells. Primary sex characteristics are structures responsible for promoting the development, preservation, and delivery of sperm cells while secondary sex characteristics are structures and behavioral features that externally differentiate men from women.
Sertoli Cells
Sertoli cells, also known as sustentacular or nurse cells, are highly specialized cells that regulate the development of spermatogonia into spermatozoa (Table 5.1). They originate from the tubular basement membrane and extend up toward the lumen of the seminiferous tubules. The basement membrane acts as a barrier that prevents large molecules in the interstitial fluid from entering the tubule but allows the entry of testosterone. Sertoli cells provide sustenance for developing spermatogonia and are involved in germ cell phagocytosis. The formation of lipid droplets in Sertoli cells is associated with this phagocytosis [9]. The number of lipid droplets found in Sertoli cells increases as the testes advance in age [10]. They also produce and secrete anti-Müllerian hormone (AMH), inhibin, activin, growth factors, enzymes, and ABP. AMH is involved in embryonic development and contributes to the regression of Müllerian ducts. Inhibin, another hormone, helps to regulate FSH secretion from the anterior pituitary. When FSH binds to high-affinity FSH receptors on the Sertoli cells, ABP is secreted (by Sertoli cells) into the lumen of the seminiferous tubule, where it binds to testosterone (secreted by Leydig cells). This causes testosterone to become less lipophilic and more concentrated within the luminal fluid.
Neighboring Sertoli cells have membrane specializations at the basolateral side that forms a band, sealing the cells together and forming a tight junction. The blood–testis barrier prevents molecules in the blood from moving past the tight junctions toward the lumen of the seminiferous tubules. This ensures that the germ cells in the later stages of development remain inaccessible to any harmful molecules in circulation.
The Blood–Testis Barrier
In the mammalian testes, the blood–testis barrier is composed of specialized junctions that are tightly bound between adjacent Sertoli cells in the epithelium of the seminiferous tubule. This barrier is also known as the Sertoli cell seminiferous epithelium barrier. The strong intercellular junctional complexes that link two adjacent Sertoli cells in the tubule form an additional barrier between the tubular lumen and the interstitial fluid outside the tubule. This divides the seminiferous tubule space into two parts: the basal (basement membrane) compartment that is in contact with blood and lymph vessels and the adluminal (lumen) compartment that is isolated from these fluids. The blood and lymph vessels and nerves are located in the interstitium between the tubules and do not penetrate the seminiferous tubules [11]. The Sertoli cells are surrounded by closely aligned myoid or peritubular cells. These arrangements collectively form the blood–testis barrier, which provides an immunologically privileged site for spermatogenesis to thrive.
The fluid found in the tubular compartment of the testes differs from that in found in the interstitium as the former contains low concentrations of glucose and high concentrations of potassium ions and steroid hormones. The tight junctions of the blood–testis barrier break and reform around the migrating cells to ensure that the barrier remains intact.
Intrinsic Regulation
The process of spermatogenesis is also regulated independently from within the testis. The Leydig cells secrete (1) testosterone, (2) neuroendocrine substances that serve as neurotransmitters, and (3) growth factors for neighboring Leydig cells, blood vessels, lamina propria of the seminiferous tubules, and Sertoli cells [12–14]. Leydig cells also contribute toward the nutrition of the Sertoli cells and help to regulate blood flow in the intertubular microvasculature [3]. The cells of the peritubular tissue influence myofibroblast contractility and regulate spermatozoa transportation via peristaltic movements of the seminiferous tubules. The Sertoli cells deliver different growth factors, and various germ cells participate in the development and regulation of other germ cells.
Spermatogenesis
Spermatogenesis is an extremely intricate process of cell differentiation, starting with germ cell (spermatogonia) development and culminating in the production of highly specialized spermatozoa. This process produces the genetic material required for species replication. Spermatogenesis occurs in the lumen of the seminiferous tubules. It was classically believed that human spermatogenesis takes about 64 days in the testis (from spermatogonium to spermatid) with an additional 10–14 days in the epididymis for maturation of spermatozoa. Thus, the entire process took about 70 ± 4 days to complete [15]. However, a more recent report suggests that the entire process from production to ejaculation of spermatozoa is completed within a shorter period: an average of 64 ± 8 days (with a range of 42–76 days) [16]. Spermatogenesis begins at puberty and occurs continually throughout the entire male adult life span in contrast to oogenesis, which is finite in women. The baseline number of precursor cells in the testes is regulated by FSH . Early in embryonic development, the gonocytes, which precede the formation of spermatogonial germ cells, undergo active mitotic replication [17] .
Spermatogenesis involves a series of cellular events that begin in the basal compartment and end in the apical compartment. The basal and the luminal compartments are kept separate by tight junctions. In the seminiferous tubules, the developing cells are arranged in a highly ordered sequence from the basement membrane toward the lumen (Fig. 5.2). Spermatogonia are positioned directly on the basement membrane. Primary spermatocytes, secondary spermatocytes, and spermatids lie closest to the lumen. Spermatogonia and primary spermatocytes are found in the basal compartment whereas secondary spermatocytes and spermatids are found in the adluminal compartment.
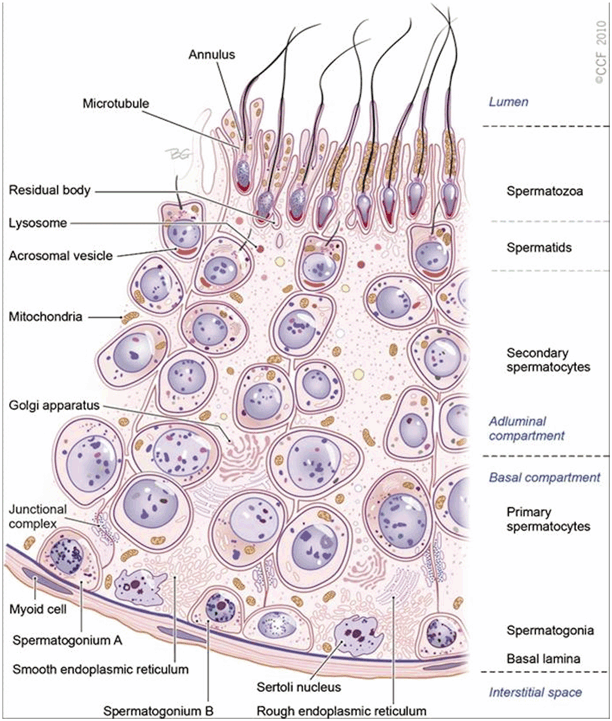

Fig. 5.2
Seminiferous tubule. A cross section of the germinal epithelium in the seminiferous tubule. The germinal epithelium is divided by the Sertoli cell into two compartments, i.e., the basal and adluminal compartments. Fully formed spermatozoa are released into the lumen. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2010–2013. All rights reserved.)
During spermatogenesis, two events occur in the basal compartment outside the blood–testis barrier: (1) the renewal and proliferation of spermatogonia via mitosis and differentiation and (2) the cell cycle progression from type B spermatogonia to preleptotene spermatocytes. The following three events occur in the adluminal or apical compartment behind the blood–testis barrier: (1) the cell cycle progression from zygotene to pachytene and then to diplotene spermatocytes, followed by meiosis I and meiosis II; (2) spermiogenesis , during which the round spermatids develop into elongated spermatids and eventually spermatozoa; and finally (3) spermiation, which involves spermatozoa maturation and subsequent release into the lumen (Table
5.2) .
Table 5.2
Terminology in spermatogenesis
Process | Description |
|---|---|
Spermatogoniogenesis | Process of producing spermatogonia through multiple mitoses to amass a large population of stem cells, most of which undergo meiosis to produce spermatozoa |
Spermatogenesis | Process of differentiation of a spermatogonium into a spermatid Purpose: to produce (via mitosis and meiosis) the necessary genetic material for species replication |
Spermatocytogenesis | Process of producing spermatocytes that occurs in the basal compartment of the seminiferous tubules |
Spermiogenesis | A complex metamorphosis that transforms round spermatids (from the final division of meiosis) into a complex structure spermatozoon |
Spermiation | Process whereby a mature spermatid frees itself from the Sertoli cell and enters the tubular lumen |
The following is an overview of the spermatogenic events. First, the primary spermatocytes undergo two meiotic divisions. The first division gives rise to two haploid secondary spermatocytes, which is followed by the second division, which gives rise to four haploid spermatids (1n, 23 chromosomes). Two of these spermatids carry the X maternal chromosome while the other two spermatids carry the Y paternal chromosome. Each spermatid will subsequently undergo spermiogenesis , a metamorphosis into spermatozoa. The spermatozoa are then released into the lumen of the seminiferous tubule (Fig. 5.3) .
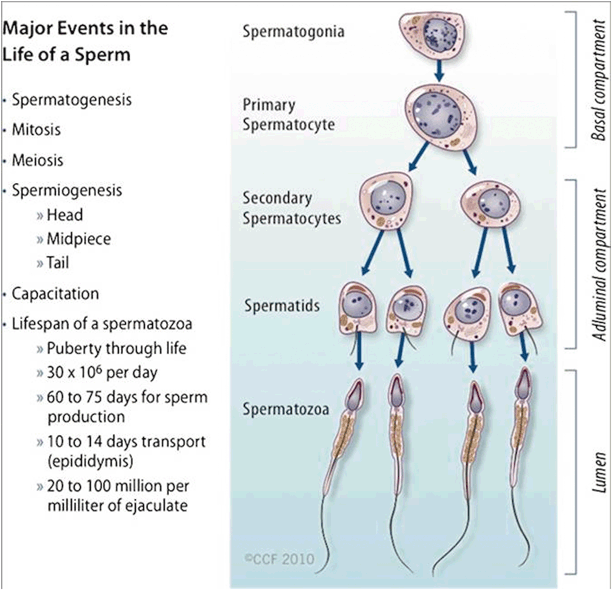

Fig. 5.3
Spermatogenesis. Major events in the life of a sperm involving spermatogenesis, spermiogenesis, and spermiation. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2010–2013. All rights reserved.)
Spermatogoniogenesis
Spermatogonia are a population of long-living primordial germ cells that undergo mitosis to provide a renewing stem cell population and meiosis for spermatozoa production. Germ cells are named according to their morphological appearance and can be categorized into two classes: Type A and Type B. In humans, Type A cells, the most rudimentary of cells, can be further classified as “pale Type A (Ap)” and “dark Type A (Ad)” spermatogonia. Ap spermatogonia can divide mitotically into more Ap cells or Type B spermatogonia. Type A spermatogonia comprise the stem cell pool whereas Type B spermatogonia continue to develop into spermatids.
Ap spermatogonia remain attached to the basal membrane and continue to replenish its numbers, allowing the spermatogenic process to persist despite the aging process. Spermatogonia continuously increase in number via successive, but usually incomplete, mitosis. On the other hand, Ad cells seldom divide, potentially serving as a dormant reserve or nonproliferative stem cells that give rise to Ap spermatogonia [15].
Type B spermatogonia have more chromatin within the inner nuclear envelope than to the intermediate or type A spermatogonia. Type B spermatogonia divide mitotically to produce primary spermatocytes, operating as differential precursors to the preleptotene spermatocytes. Spermatogonia remain joined by intercellular bridges but dissolve in the advanced phases of spermatid development. The synchrony of germ cell maturation is thus maintained [18], which is likely to aid in its biochemical interactions.
Spermatocytogenesis
Spermatocytogenesis involves the formation of spermatocytes and takes place in the basal compartment of the seminiferous tubule. The process begins with the primary spermatocytes undergoing meiosis I to form secondary spermatocytes. The prophase of the first meiotic division is very long and thus, the primary spermatocyte has the longest lifespan. Secondary spermatocytes then undergo the meiosis II to produce spermatids. Secondary spermatocytes have a comparably shorter lifespan of 1.1–1.7 days .
Spermatogenesis , from spermatogonium division to spermatozoa release into the tubule, takes about 64 days to complete. Sperm released into the lumen of the seminiferous tubules are immature and incapable of moving on their own. They are pushed through the lumen both by other developing sperm cells moving toward the lumen and by the bulk flow of fluid secreted by Sertoli cells. Sperm cells entering the epididymis complete maturation after 10–14 days of transit, aided by protein secretions from epididymal cells.
Disruption of Spermatogenesis
Type A spermatogonia are necessary for spermatogenesis , and in cases of reduced spermatogenesis, it is likely that Ad spermatogonia are absent [8]. When Type A or Type B spermatogonia are absent and the germinal epithelium is made up only of Sertoli cells, then spermatogenesis will not occur. This “Sertoli Cell Only Syndrome” may be congenital (absence of spermatogonia from birth) or acquired (spermatogonia destroyed by exposure to radiation, etc.). Spermatogenic arrest at the spermatogonial stage occurs when Ap spermatogonia fail to develop into Type B spermatogonia [19].
Mitosis (Cytodifferentiation of Spermatids)
Mitosis involves nuclear division and separation of duplicated chromosomes to form two daughter cells with genetic content exactly identical to its parent cell (diploid, n = 46). Mitosis is vital for proliferation and maintenance of spermatogonial cells. Meiosis involves an intricate series of events that encompass the duplication of chromosomes, nuclear envelope breakdown, and equal division of chromosomes and cytoplasm that leads to the formation of two daughter cells. Specific regulatory proteins interact on DNA loop domains during cellular replication [20, 21]. The germ cells involved in the mitotic phase are the Type A spermatogonia, which first form the Type B spermatogonia and later the primary spermatocytes. Through a series of mitotic divisions, developing germ cells, which are interconnected by intracellular bridges, produce primary spermatocytes—the largest germ cell of the germinal epithelium. The baseline number of spermatogonia is established after puberty. Mitosis then supplies the precursor cells and initiates the differentiation and maturation processes.
Meiosis
Meiosis is a complex process during which chromosomal exchange of genetic material occurs to form four daughter cells with half the number of chromosomes (haploid, n = 23) compared to their parent cells. The purpose of meiosis is to ensure genetic diversity. The germ cells involved in the meiotic phase are the primary spermatocytes, secondary spermatocytes, and spermatids. Meiosis occurs twice in succession as meiosis I and meiosis II; each meiotic process consists of prophase, metaphase, anaphase, and telophase. Prophase itself is made up of four stages: leptotene, zygotene, pachytene, and diplotene. Leptotene takes place in the basal compartment while the remaining three take place in the adluminal compartment. Meiosis I is the reducing division in which the number of chromosomes are halved (i.e., the replicated chromosomes in one cell is split between two diploid cells). Meiosis II is the division in which there is no DNA replication and the sister chromatids are split, resulting in four halpoid cells.
The meiotic process is regulated by its own specific mechanisms [22]. In the seminiferous tubules, meiosis begins with the detachment of Type B spermatogonia from the basement membrane to form preleptotene primary spermatocytes. In theory, each primary spermatocyte yields four spermatids, but the actual yield is lower as some of these germ cells are lost in the process. After meiosis I, each daughter cell (secondary spermatocyte) contains one half of the homologous chromosome pair. The secondary spermatocytes then quickly undergo meiosis II, during which time the chromatids separate at the centromere, yielding early round spermatids with haploid chromosomes “22X” or “22Y.” During the entire meiotic phase, homologous chromosomes pair up, cross over, and exchange genetical material to form an entirely new genome . Defects during meiosis include apoptotic spermatocytes and spermatogenic arrest of primary spermatocytes. These germ cells bordering the seminiferous tubules cease to develop further and disintegrate [8].
Spermiogenesis
In spermiogenesis , haploid spermatids undergo complete differentiation or morphogenesis to form highly specialized spermatozoa with fully compacted chromatin. These morphological changes begin after meioses I and II. In humans, there are eight different stages (Sa-1, Sa-2, Sb-1, Sb-2, Sc-1,Sc-2, Sd-1,and Sd-2) involved in the maturation of spermatids to spermatozoa. Each stage is identifiable by the maturing cell’s morphological characteristics. In the postmeiotic phase, there is progressive condensation of the nuclear chromatin (to about 1/10 the volume of an immature spermatid) with the inactivation of the genome. In addition, the Golgi apparatus forms the acrosome cap, and the flagellum structures begin to develop [8]. Histones—alkaline proteins that condense the DNA—are converted into transitional proteins, and protamines are converted into well-developed disulfide bonds. Defects during spermiogenesis include acrosomal and flagellar defects, absence of the acrosome or the midpeice of the flagellum, and impaired nuclear condensation in malformed spermatids [8] .
Nuclear Development
The nucleus and its contents undergo several changes during spermatogenesis . During the first eight steps of spermiogenesis [23], the nucleus elongates and flattens, giving the head its characteristic oval shape. This nuclear compaction is believed to facilitate oocyte penetration and help to optimize spermatozoa swimming capacity [24]. This nuclear compaction includes chromatin remodeling. During the last postmeiotic phase of spermiogenesis , histone molecules, around which DNA is organized, are converted to translational proteins that are then converted to protamines [25]. Protamines contain large amounts of cysteine, which aids in disulfide bond formation as the sperm cells mature in the epididymis [26–28]. Protamines in the chromatin of the spermatozoa are replaced by histones from the oocyte within 2–4 h of fertilization .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





