Fig. 13.1
An ileal GIST: at abdominal CT in the axial scans (a) and at CE (b)
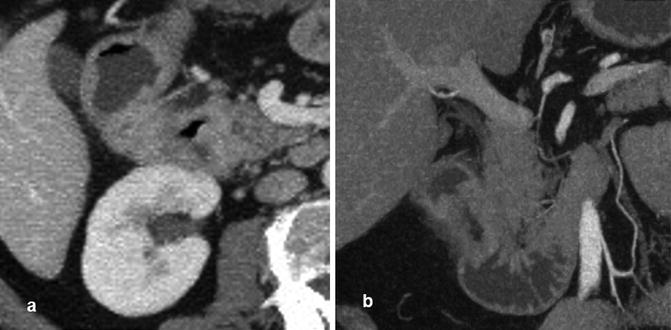
Fig. 13.2
CTE showing, in axial scan, a duodenal lesion (a) and, in coronal scan, neoplastic stenosis with thickening of the wall (b)
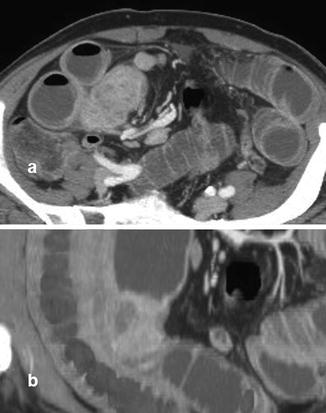
Fig. 13.3
Ileal carcinoid, in CTE axial (a) and coronal (b) scan, appearing like a solid formation with inhomogeneous enhancement
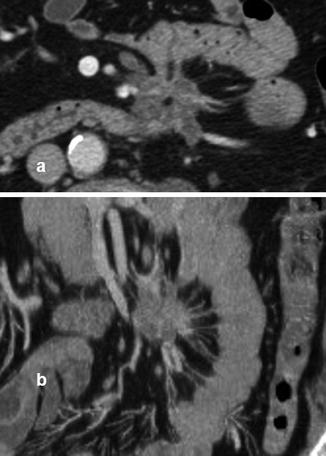
Fig. 13.4
Intestinal lymphoma in patient with HIV at the abdomen CT in axial (a) and coronal (b) scan
SBTs typically present in middle-aged adults and elderly people, especially during the sixth and seventh decades, with a slight male predominance. There is a geographical variation with SBTs being more frequent in Western countries, as well as a racial/ethnic difference, with a higher incidence of adenocarcinomas and malignant carcinoids among black populations [2–4]. Although a complete understanding of the pathogenesis of SBTs has not yet been reached, it is generally postulated that the small bowel has some protection against cancer that the adjacent organs lack [4]. In particular protective factors may be rapid cell turnover, rapid transit times, low-bacterial load, alkaline environment, low levels of activating enzymes or precarcinogens, high levels of lymphoid aggregates, and IgA. Environmental and behavioral factors may certainly also play a role. Finally, some medical or genetic cancerous and noncancerous bowel conditions, such as celiac disease, inflammatory bowel disease, inherited polyposis (discussed later in this chapter) and nonpolyposis syndromes, confer an increased risk for developing SBTs [3, 4]. Hence, patients presenting with SBTs should always be assessed for one of these underlying conditions [5].
As for secondary small-bowel cancers, they can occur either by direct invasion or as a result of distant metastasis spread. Metastatic SBTs derive from melanomas, prostate, lung, kidney, breast, and testes. Melanoma is the most common metastatic tumor to the small bowel. Intestinal melanomas can be primary tumors but more commonly are metastases, affecting the jejunum and ileum [6] (Fig. 13.5, Video 13.1).
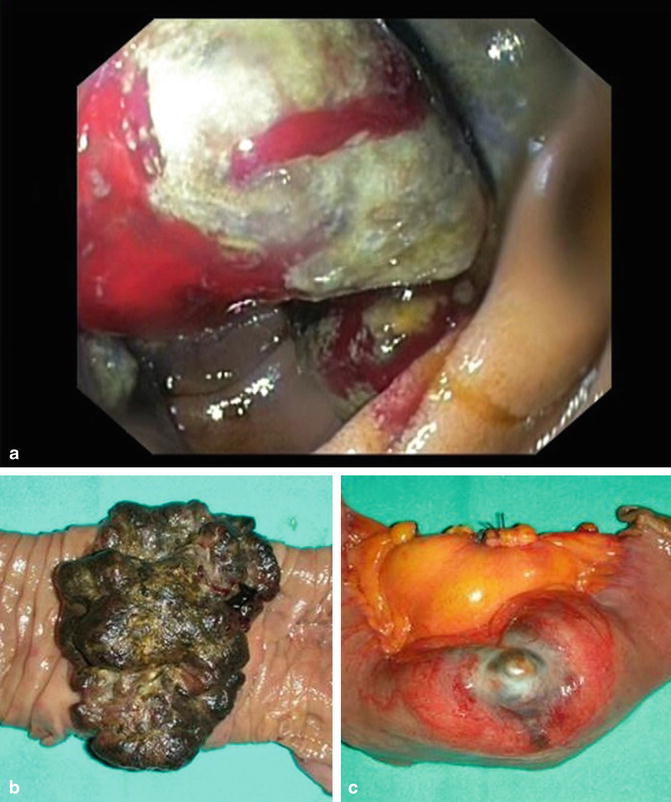

Fig. 13.5
Melanoma of the small bowel, at endoscopic diagnosis (a) and surgical specimen after resection (b, c)
The rising incidence rates of primary SBTs [2] partially reflect both a better understanding and increased awareness of the disease, with a higher index of clinical suspicion and a lower threshold to order further investigations. It is also related to the improvements in diagnostic capabilities with the advent of novel endoscopic devices and high-performance radiological techniques for a thorough small-bowel evaluation. Due to their rarity and nonspecific clinical presentation, SBTs are indeed difficult to diagnose and quite often detected in advanced stages. Physical examination is usually unrevealing. Symptoms and signs associated to SBTs are vague, nonspecific, indistinct, or inconclusive. They may include any of the following: dyspepsia, minor anemia, vague or cramping abdominal pain, bloating, fatigue, nausea and vomiting related to partial, intermittent, or complete bowel obstruction, weight loss, anorexia, diarrhea, perforation, palpable abdominal mass, jaundice in case of periampullary lesions, frank bleeding, and chronic obscure-occult bleeding. All of these clinical signs and symptoms show poor sensitivity and specificity. Even the serotonine activity-related visible signs and symptoms of carcinoid syndrome (flushing, diarrhea, wheezing) correlate with a tumor that has already progressed [6–9].
Malignant tumors arising from the small bowel have a poor prognosis, with an overall 5-year relative survival rate of 54 % (83 % for carcinoids, 25 % for adenocarcinomas, 62 % for lymphomas, and 45 % for sarcomas) [10]. Many SBTs can remain clinically silent for years. The available literature reports approximately a 10- to 20-month mean delay in diagnosis from first symptoms. Delayed diagnosis contributes to the poor survival prognosis. Diagnosis at an early stage would be desirable, because the mainstay of treatment for adenocarcinomas, GISTs, and carcinoids is complete curative resection. Cancer-directed surgery, early stage disease, and lymph node involvement ratio are key prognostic factors significantly associated with overall survival. On the other hand the diagnosis of a lymphoproliferative disorder may change the management from surgical to medical [11–14]. All of these factors support the need to have a high index of suspicion for small-intestinal neoplasms and to perform a more aggressive diagnostic work-up in patients with vague gastrointestinal symptoms (Video 13.2).
SBTs in the Old Era
Until the advent of capsule endoscopy and device-assisted enteroscopy coupled with the advances in radiology, physicians had to deal with limited diagnostic capabilities for investigating the small bowel. Accurate and early preoperative diagnosis of SBTs proved difficult. Intraoperative enteroscopy (diagnostic yield ranging from 70 to 100 % in patients with obscure-gastrointestinal bleeding) and surgery were the sole, rather invasive, procedures providing definite evidence of lesions, polyps, or masses deeply located in the small intestine and allowing their treatment [15]. Leaving the obsolete sonde enteroscopy out of any consideration, diagnosis of SBTs was mainly based on barium-contrast radiology, such as small-bowel follow-through (SBFT) (Fig. 13.6) or enteroclysis, push enteroscopy, or in selected cases angiography. Although abnormalities may be seen in up to 83 % of patients with SBTs, upper gastrointestinal series with a SBFT deserves limited utility given its low sensitivity for SBTs of 30–44 % [16, 17]. Owing to their partial evaluation of the small bowel and overall low-diagnostic yield, these techniques are gradually being phased out in favor of more efficient and effective endoscopic and radiological techniques.
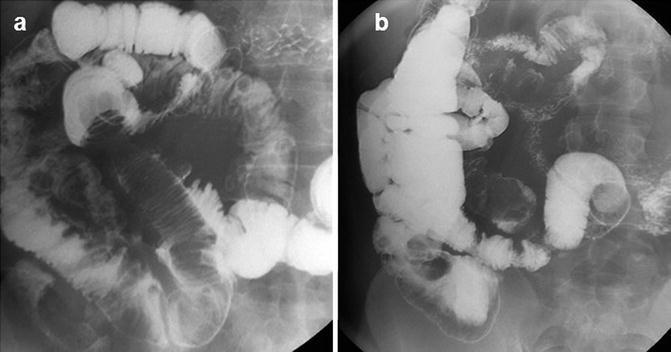

Fig. 13.6
Ileal lipoma with intussusception at SBFT: filling defect with spiral morphology in ileal loop
Regardless of clinical indications, sensitivity at enteroclysis is superior to sensitivity at SBFT: 87–96 % reported by most authors [18]. In particular, enteroclysis is a good technique to identify folds, masses, and large protruding proximally located or obstructive small-bowel lesions. In a series of 71 patients diagnosed with primary mesenteric malignant tumors of the small intestine over a 21-year period, the tumor detection rate of standard enteroclysis was 90 % as compared to 33 % of SBFT [19]. However, surface coating is suboptimal due to barium dilution and enteroclysis is ineffective in visualizing the luminal surface directly, small sized and flat lesions, and/or lesions of the terminal ileum. Moreover, patient’s discomfort, high-radiation dose, and complexity have limited its use [20].
Conventional computed tomography (CT) scan and magnetic resonance (MR) imaging are reliable methods for detection of small-bowel masses, for localization, for assessment of their relationship to adjacent structures, and for staging of metastatic spread and lymph nodes involvement. However, it is important to note that they might miss small intraluminal and mucosal lesions and lack the capacity to distinguish subtypes of SBTs [18, 21]. Over the past years, conventional CT scan and MR imaging have substantially been improved with the adjunct of oral or small-bowel contrasts allowing a more accurate bowel filling and distension, and demonstrating high accuracy for the detection and location of small-bowel lesions [22, 23].
Push enteroscopy is limited to the proximal jejunum, allowing an average of 50–100 cm of the small intestine to be intubated. It is still useful for the identification and sampling of proximal tumors, but is inferior to CE and push-and-pull enteroscopy with regard to the length of the small bowel visualized, as well as the diagnostic yield [24, 25]. In patients referred for obscure-gastrointestinal bleeding (OGIB) and positive radiologic findings, the diagnostic yield of push enteroscopy for SBTs is about 5–6 % [26]. Standard esophagogastroduodenoscopy plays a primary role in the assessment of lesions located in the duodenum. The terminal ileum is successfully investigated through colonoscopy with ileoscopy up to 45 cm [27].
SBTs: Outcome and Results in the New Era
Since the advent of capsule endoscopy (CE) there has been a growing body of evidence showing its superiority over the traditional small intestine diagnostic tests. CE proved superior to SBFT and push enteroscopy for the diagnosis of small-bowel diseases. CE effectively identifies lesions beyond the reach of push enteroscopy, including SBTs undetected by conventional radiological studies with a diagnostic yield of 52.6–65.2 % [25, 28, 29]. As such, CE has become the frontline diagnostic tool in case of suspected small-bowel diseases, after negative upper GI endoscopy and colonoscopy [30]. However, it is important to emphasize that CE, though providing a complete evaluation of the small bowel, has limitations. In particular, CE is not very good at evaluating the duodenal sweep and the periampullary area. Standard endoscopy methods are still important here [31, 32]. A possible explanation is the rapid capsule transit time across the duodenal sweep.
Clinical studies on CE have reported a frequency of SBTs differently ranging between 4 and 10 %. The majority (approximately 60 %) are malignant. Occasionally, small-bowel malignancies are secondary tumors, mainly originating from skin melanomas [29, 33–38]. The great variation in the reported frequency of SBTs is likely multifactorial and related to the retrospective design of the studies, the analysis of prevalence only in patients with OGIB, the absence of a standardized approach, the inclusion of both benign and malignant tumors [37], and the inclusion of hereditary polyposis syndromes. In any case, it appears that CE has increased the rate of diagnosing SBTs and a detection rate even up to 13.7 % has been reported in the recent literature [39]. This might be the result of a selection bias in tertiary referral centers. This is supported by the fact that a recent multicenter European study [40] including 5,129 patients undergoing CE showed a 2.4 % frequency of SBTs. This surprisingly low result seems consistent with the 1.6 % detection rate of SBTs reported in another large study based on 1,000 CE examinations [41]. The low frequency of tumors detected might be related to the high number of capsule studies performed, as the authors themselves suggest [40].
Chronic occult or overt bleeding is the most common presentation of SBTs [15] and the usual clinical indication for CE in 80 % of cases [29, 35, 37, 40]. Whenever CE is performed for OGIB, SBTs are detected in 6–12 % of cases, the incidence being higher in adults <50 years of age [6, 15, 42]. In descending order of incidence, SBTs reside in the jejunum in 40–60 % of cases, in the ileum in 25–40 % of cases, and less frequently in the duodenum (15–20 % of cases) [29, 33, 35–37, 41]. The preponderance of the lesions in the mid-gut may account for the extensive work-up that patients usually have undergone prior to CE. The literature suggests that patients undergo between 3.6 and 5 negative procedures, including SBFT, enteroclysis, push enteroscopy, and abdominal CT scan [33, 37, 43]. As for the endoscopic appearance of SBTs at capsule imaging, many descriptors have been used to describe the findings [39]. The heterogeneous terminology used for capsule images has prompted an expert panel to establish a structured common terminology, which includes nodule, polyp, submucosal, ulcerated, fungating, frond-like or villous, bleeding or nonbleeding mass, or tumor [44, 45]. SBTs generally appear at CE as masses or polyps in 70–80 % of cases and as ulcers or strictures in 20–30 % of cases [46].
Based only on capsule images, it is not possible to distinguish the tumor type or whether a lesion is malignant or benign [46, 47]. Therefore, in case of a suspected SBT—for example, as a result of cross-sectional imaging techniques—the European Society of Gastrointestinal Endoscopy (ESGE) guidelines on flexible enteroscopy recommend balloon-assisted enteroscopy as the first choice, given its potential for histopathological diagnosis through tissue sampling [47]. A novel index aiming to overcome the potential CE false-positive findings and aiming to discriminate a mucosal bulge from a mass was recently developed: the Smooth Protruding Index on Capsule Endoscopy (SPICE) score [48]. A SPICE score higher than 2 had an 83 % sensitivity and 89 % specificity for tumors. Other attempts to differentiate tumors on CE include an automated scale using multiscale wavelet-based analysis in capsule endoscopy images that is reported to have 93 % sensitivity and specificity [49].
CE allows noninvasive evaluation of the entire small bowel in 79–90 % of patients, with a high-diagnostic yield and positive and negative predictive values approaching respectively 83, 97, and 100 % in the evaluation of OGIB [50]. The performance of CE for tumor detection was very good with sensitivity, specificity, NPV, and PPV values reaching 83.3, 100, 97.6, and 100 %, respectively [51]. In the setting of SBTs, the influence of CE on the final diagnosis and management may be as high as 77 % [43]. With regard to therapeutic impact, in one series including 443 CE examinations with a SBT detection rate of 2.5 %, CE had an impact on therapy in 6 out of 11 patients (55 %) [37]. In a large-multicenter study on 1,132 patients, where 4.3 % (57) of SBTs were diagnosed with CE, capsule critically changed the therapeutic course in 12.3 % of patients (7/57) leading to surgical intervention [28]. In the study by Bailey et al., curative resection was performed in 52 % of patients with SBTs and they remained recurrencefree at a mean follow-up of 38 months, most likely because of an earlier stage at diagnosis [29]. Further long-term studies are needed to clarify the impact of CE on outcomes and its role in surveillance of SBTs.
Capsule endoscopy can also play a role in surveillance of small-bowel lesions. CE has been shown to be useful in the surveillance of small-bowel lymphomas and to assess response to treatment, including lymphomas in type II refractory celiac disease [52, 53]. The ESGE guidelines indicate, in these cases too, the importance of balloon-assisted enteroscopy as for the ability to take biopsies [47].
CE is actually a mere visual diagnostic tool. Its major drawbacks include the inability to perform tissue sampling, the absence of therapeutic capabilities, the inability to control its movement through the gastrointestinal tract, and poor localization and sizing of lesions. Another important disadvantage is the misdiagnosis due to false-negative CE examinations [54]. Poor bowel preparation, rapid passage, random movements of the capsule, inadequate bowel distension, or incomplete small-bowel examinations may hamper or prevent diagnosis. The highest miss rate refers to solitary small-bowel mass lesions. In a pooled analysis of 24 trials representing 530 patients, the authors recorded an 18.9 % CE miss-rate for small neoplasms [55]. Recent comparative studies reported on SBTs missed at CE but identified with CT enterography [56, 57] or balloon-assisted enteroscopy (BAE) [58], with two-thirds of the overlooked SBTs involving the duodenum/proximal jejunum [51, 57]. When stratified according to the site of tumor, the diagnostic yield for SBTs in the distal duodenum/proximal jejunum is lower (73 %) than for those located more distally (90 %) [57]. Though a negative CE may be reassuring in many cases, when there is a high index of suspicion, as in the case of persistent or alarm symptoms, the clinician should have a low threshold to further investigate with complementary endoscopic and/or radiologic tests [56–59].
This must also be balanced by the fact that CE can identify nonspecific lesions that are, in fact, false positives. Incidental findings, such as erosions and angiodysplasias, are common and may be mistaken as a source of bleeding [46, 50, 56]. Another limitation of CE is the possible risk of retention. The risk of CE retention due to a stricture or mass, in the setting of a definite or suspected SBT, may be as high as 10 % [40]. A pooled analysis of 227 papers including 22,840 CE procedures found an overall CE retention rate of 1.4 % [60]. A low-retention rate was associated with OGIB, whereas a relatively high retention rate was associated with neoplasms. In many of these cases, however, CE retention may be regarded as a therapeutic adverse event, leading to a diagnosis and ultimately to surgical resection of the lesion and CE retrieval. The use of a dissolvable patency capsule to test for small-bowel patency is a viable method to reduce retention risk in patients at higher risk for obstruction [46].
Following the development of the capsule, the advent of device-assisted enteroscopy (DAE) is a further major breakthrough in the diagnosis and therapy of small-bowel cancerous lesions. DAE encompasses double-balloon enteroscopy (DBE), single-balloon enteroscopy (SBE), and spiral enteroscopy (SE). All of these techniques allow a deep and theoretically total enteroscopy with the assistance of an overtube that facilitates the pleating of the small bowel over the scope and overtube assembly by means of push-pull anchoring movements or rotational movements. Using these techniques, the depth of intubation of the small bowel (240–360 cm on oral approach and 102–140 cm with the retrograde route) is much greater compared to push enteroscopy and ileoscopy [61]. The rate of total enteroscopy has been reported to range from 5 to 86 % and is very operator dependent [62]. Patients having undergone extensive abdominal surgery may be poor candidates for DAE because of adhesions or altered anatomy, which may prevent the endoscope from advancing. The subtypes of DAE share the same indications, drawbacks (invasive nature, prolonged duration, requirement for additional personnel), and complications (pancreatitis, perforation, bleeding, paralytic ileus) without significant differences in technical and clinical success rates [61]. The choice of one or the other technique depends on local expertise and availability. DAE is invasive and labor intensive, but holds the potential for histological diagnosis with tissue sampling. Moreover, it has the ability for therapeutic interventional procedures including polypectomy, hemostasis, palliative dilation, stent placement, tattoos for SBT detection at minimally invasive surgery (Video 13.3), and retrieval of retained capsules [61, 63]. In the setting of SBTs, DAE provides the ability to diagnose, apply therapy, and precisely localize the lesion. Therapeutic maneuvers, however, do raise the complication rate from 1 to 4–5 % [64, 65].
As for CE, the main indication for DAE is OGIB [66–68]. However, DAE is increasingly used to confirm abnormal CE or cross-sectional imaging findings, to evaluate patients with celiac disease for the presence of enteropathy-associated T-cell lymphoma, or to screen or survey patients with intestinal polyposis syndromes (discussed later) [66, 68]. Since the advent of DBE in 2001 many papers addressing the diagnosis and management of SBTs have been published. In the largest series from a Japanese multicenter study (a retrospective analysis of 1,035 patients undergoing DBE for various indications), 13.9 % of patients were found to have SBTs, with 7.3 % primary cancers detected in nonpolyposis patients [68]. In most of the cases the diagnosis was made through DBE and in some cases a therapeutic procedure was directly performed. The most common indication for DBE was OGIB (44 %), followed by obstructive symptoms inclusive of abdominal pain (12 %), evaluation or treatment of diseases such as inherited polyposis syndromes (12 %), the suspected presence of a SBT (9 %), and other indications (23 %) [68]. In this study, as in other studies [67, 69, 70], the incidence of SBTs in patients undergoing DAE is significantly higher than reported in CE studies. The current literature, mostly based on retrospective series, shows heterogeneous results in SBT detection rates by DAE, ranging from 3.6 to 17.4 % [58, 66–71].
The reasons for a high variation in detection rates for SBTs is probably multifactorial, including selection bias. The diagnostic yield of DBE appears to be highest in patients who have positive findings on previous radiologic studies, CE, or octroescan [72]. Many series include benign tumors or patients known to have genetic disorders (Familial Adenomatous Polyposis or Peutz-Jeghers syndrome) where the incidence of SBTs may be expected to be higher or patients may be specifically referred to evaluate and treat specific lesions, as in Peutz-Jeghers syndrome. Smaller studies where inherited polyposis and benign tumors were excluded have indeed reported a surprisingly low (3.6 %) SBT incidence in patients examined by DBE [66]. Finally, the differences may partially reflect a dissimilar referral practice to tertiary referral centers or a true geographic or ethnic patient population difference in the incidence of the various types of SBTs. In the Japanese series [68], lymphomas and GISTs (Figs. 13.7, 13.8, and 13.9) were found more commonly. Neuroendocrine tumors (NETs) were rarely diagnosed (2.8 %), whereas in the West, based on a 5-year experience at a US referral center, there was a higher incidence of NETs (40 %) [66]. Carcinoid tumor was also reported to be the most common small-bowel cancer identified by DBE in the US study by Cangemi et al. [69]. This geographic variation needs to be further investigated.
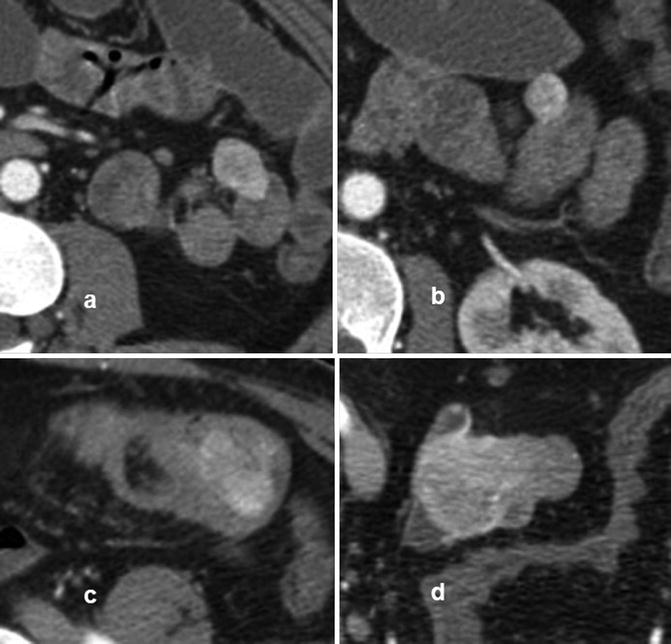
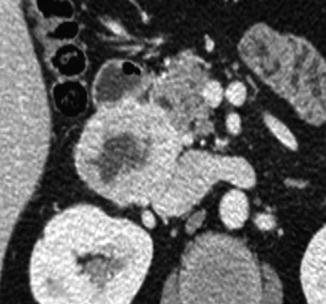
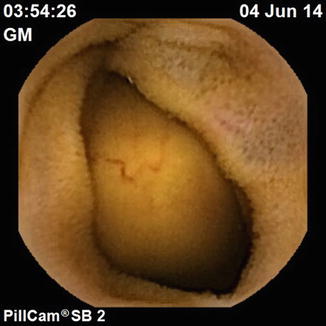

Fig. 13.7
CTE with evidence of multiple jejunal (a, b) and ileal (c, d) GISTs

Fig. 13.8
CTE with evidence of duodenal GIST

Fig. 13.9
CE finding of jejunal GIST
There is some information on the comparable diagnostic yield between CE and DAE. A meta-analysis by Pasha et al. found the diagnostic yield for small-bowel lesions to be equal between DBE (57 %) and CE (60 %) [73]. However, as already pointed out, DBE may identify small-bowel mass lesion undetected on CE [58]. Some studies demonstrated DBE to be superior to CE, and CE achieving comparable diagnostic yield compared to DBE only when combined with contrast-enhanced CT (91 % versus 99 %) [59]. On the other hand, tumors may be missed on DBE as well after a positive CE result [74, 75], usually because of their location in an area of the small bowel not examined by DBE.
CE and DAE have to be viewed as complementary tests for the evaluation of patients with suspected SBTs and CE is considered the preferred initial test of choice [76]. CE is a noninvasive test, while DAE is time consuming and more invasive. If no lesions are found on CE, one may avoid the need for an invasive procedure. However, if a lesion is detected, CE can direct the route for a “targeted DAE” and guide the selection of the intubation approach. For deep intubation, according to findings and transit times, the oral approach is preferred when the lesion is suspected to be within the proximal 75 % of the small bowel, whereas a retrograde route is used for more distal lesions [76–78]. If CE is negative but there is a high index of suspicion, further investigations with DAE or cross-sectional imaging should be pursued [56–59]. It should also be mentioned that repeat CE may also identify additional findings in up to 75 % of patients with OGIB, leading to a change in management in 62 % of cases [79]. In some cases, there is a role for deep enteroscopy as the first-diagnostic test without a prior CE, especially when there is a high index of suspicion [63] and/or there is a risk of obstruction and capsule retention [68].
The clinical impact of DAE on the management of small-bowel cancers is illustrated by the high percentage of patients who undergo surgery based upon the findings at DAE [66]. In the series of Lee et al. [67] the results of DBE affected the surgical and endoscopic therapeutic plans and short-term clinical results among patients with SBTs in 64 % of cases. The therapeutic plans were changed more frequently in patients with SBTs than patients with other conditions. Based upon the surgical results, DAE also proved extremely accurate for locating small-bowel neoplasms and for histological diagnosis [69, 70]. DBE was very effective for enteroscopy-guided self-expandable metal stents placement for rescue palliation of malignant small-bowel obstruction with a technical success rate of 94.7 % [80]. Novel areas of innovation and potential application include the use of endoscopic ultrasound for evaluation of SBTs. DAE-guided endoscopic ultrasonography offers adequate imaging and useful information on the wall structure and nature of the detected abnormalities in the deep parts of the small bowel. These high-resolution cross-sectional images of SBTs may be helpful in the differential diagnosis of these submucosal tumors [81, 82]. However, further large-scale patient population studies and prospective studies are needed to assess the impact of DAE on long-term outcomes in patients diagnosed with SBTs and to further define its role in the diagnosis, management, and surveillance of treated tumors. In the majority of the studies, complete follow-up was not obtained as the patients following DAE were returned to the primary referring institution or physician [66, 69].
What is the contribution of radiology in this renewed and revolutionized panorama of small-bowel endoscopy? Barium enteroclysis has demonstrated reasonable accuracy in the detection of SBTs and has until recently been the radiological option of choice. However, it is unable to assess the mural and extramural extent of the disease. Despite improvements in multidetector CT, conventional CT scans detect only large-sized intestinal tumors greater than 1.0 cm in diameter. On the other hand, CT enteroclysis/enterography (CTE) provides not only excellent images of the luminal side but also information about mesentery, perienteric fat, lymph node status, and adjacent organs with 100 % sensitivity and 95 % specificity. CTE can detect tumors that are only 5 mm in diameter and the negative and positive predictive values for carcinoid tumor in patients with carcinoid symptoms were 100 and 94.7 %, respectively. CTE allows for the assessment of extraluminal disease and potential metastatic spread, and helps in preoperative staging [83, 84]. MR enteroclysis/enterography (MRE) offers the advantage of soft tissue contrast and multiplanar imaging without radiation exposure. MRE is able to identify small-intestinal strictures, especially in tumors with intestinal obstruction, based on the signal difference generated by the intestinal wall and luminal contrast agents, and shows very high overall diagnostic accuracy of 95 % for SBTs [85] (Fig. 13.10).
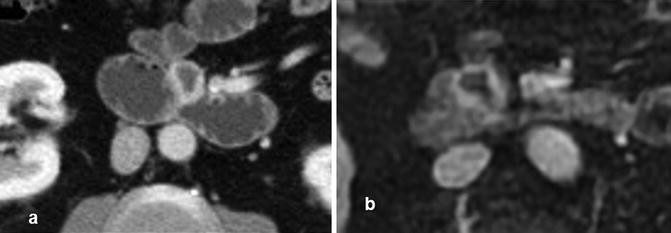

Fig. 13.10
NET at CTE (a) and at a MRE (b) showing soft tissue contrast
Small-Bowel Polyps and Polyposis Syndromes
Sporadic Duodenal Adenomas, Familial Adenomatous Polyposis (FAP), Peutz-Jeghers Syndrome (PJS), and Hamartomatous Polyps: Basic Outlines
Polyps of the small bowel are a rare entity compared to polyps of the colon. They can be sessile or pedunculated and, according to histology, divided into inflammatory, hyperplastic, hamartomatous, and adenomatous polyps. Hamartomatous polyps and adenomas may arise sporadically or be associated with polyposis syndromes. Small-bowel polyps need to be correctly recognized and histopathologically defined for adequate management, treatment, and follow-up [86].
The duodenum can easily be investigated with conventional upper GI endoscopy. Brunner gland hyperplasia is commonly seen in the context of peptic duodenitis, appearing as nodular duodenitis. In most cases no intervention is necessary but it may deserve treatment when causing problems such as hemorrhage, intestinal obstruction, intussusceptions, or is associated with suspicious lesions [87]. Sporadic duodenal adenomas are an uncommon incidental finding during upper GI endoscopy and are found in up to 5 % [88]. They are usually asymptomatic. Whenever possible, sporadic adenomas should be removed with endoscopic resection, which is preferred over surgical intervention [89]. There is no evidence to support surveillance endoscopy or regular follow-up of these lesions, especially in elderly patients or patients with relevant comorbidity [90]. However, there is accumulating data based on retrospective cohort or case-control studies, suggesting a clinically important association with colorectal neoplasia [91]. Colonoscopic assessment is thus advisable in all patients diagnosed with sporadic duodenal adenomas [92]. The broad category of intestinal polyposis syndromes embraces familial adenomatous polyposis (FAP), as well as hamartomatous polyposis syndromes. The hamartomatous polyposis syndromes consist mainly of Peutz-Jeghers syndrome (PJS), PTEN-associated hamartomatous syndromes (including Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome [BRRS]), juvenile polyposis, Cronkhite-Canada syndrome, and hereditary-mixed polyposis syndrome (HMPS). The two most common inherited intestinal polyposis syndromes are FAP and PJS [92]. As already mentioned, the clinically most relevant classification of polyposis syndromes relies on the histological typing of polyps, which is crucial for their diagnostic work-up and further management.
FAP and its phenotypic variants are an autosomal dominant inherited disorder caused by germline mutations in the oncosuppressor adenomatous polyposis coli (APC) gene, located on chromosome 5 (segment 5q21-q22). Classic FAP is characterized by the growth of hundreds to thousands of synchronous adenomatous polyps throughout the large bowel during childhood and adolescence. There is virtually a 100 % lifetime risk of colorectal cancer (CRC) by the age of 40, requiring early prophylactic colectomy before the age of 25. Attenuated FAP (AFAP) is characterized by fewer polyps at presentation, with a tropism to the proximal colon, lower penetration of cancer, and later onset of CRC [92]. Most polyposis syndromes share intestinal extracolonic and extraintestinal features. Besides colon cancer, FAP patients exhibit a higher risk of developing small-bowel adenomas and carcinomas, especially in the duodenum, and periampullary region. Adenomas in the duodenum can be found in 50–90 % of cases, with a lifetime risk of duodenal cancer up to 5 %, as highlighted by three prospective studies on duodenum surveillance [93–95]. Not unusual is the development of ileal pouch adenomas and even cancer after proctocolectomy, correlating with duodenal polyposis and with mutations involving exon 15 of the APC gene [96].
The polyposis associated with the mutation of the gene MUTYH (MUTYH-associated polyposis [MAP]), located on chromosome 1, is a recessive autosomal condition. The clinical presentation is usually similar to AFAP, but the condition can also mimic classic FAP. Data regarding intestinal extracolonic tumors are scarce: duodenal polyposis seems to be less frequent (reported in up to 17 % of patients) and the risk of developing a duodenal cancer is unknown [97]. To date, given the predominant descriptive and retrospective studies, guidelines for the management of FAP and MAP patients are mainly based on expert opinion recommendations [98] in which the clinical usefulness of a systematic small-bowel screening and surveillance program has yet to be determined. Current guidelines recommend scheduled standard upper endoscopic surveillance in relation to the Spigelman classification to investigate the duodenal polyposis, though there is no consensus at which age to start. Based on expert opinion it is advisable to start between the ages of 25 and 30 [98]. Furthermore, current guidelines suggest endoscopic follow-up of the remnant rectum after colectomy with ileorectal anastomosis and of the pouch after proctocolectomy with ileal pouch-anal anastomosis respectively at 3- to 6-month and 6- to 12-month intervals [98].
Peutz-Jeghers is an autosomal dominant inherited polyposis disorder most frequently (80–90 % of cases) arising from a germline mutation of the serine/threonine kinases gene (STK11), located on chromosome 19 (segment 19p13.3). This disorder is characterized by characteristic hamartomatous gastrointestinal polyps, mainly involving the small bowel (60–90 %), in association with mucocutaneous pigmentation. The natural history of the disease is associated with repeated polyp-related complications, consisting of intussusception, obstruction, or bleeding, often requiring endoscopic polypectomy or surgical resection [99, 100]. The overall risk of developing a cancer at any site is significantly high, with an estimated 15-fold increased risk compared to the general population, as reported by Giardiello et al. in a meta-analysis involving 210 patients from 79 families with PJS [101]. Specifically, PJS patients are at risk of developing stomach, small bowel, colon, pancreas, and breast cancer, due the hamartomatous polyps harboring adenomatous dysplastic foci, as observed in 3–6 % of the removed hamartomas. Lifetime incidence for small-bowel cancer reaches 13 %. The increased risk for cancer is age dependent and moreover the risk exponentially increases after the age of 50 [101–103]. The small-bowel tropism of Peutz-Jeghers polyps makes their detection and treatment a challenging issue. Current guidelines recommend scheduled small-bowel surveillance with CE or MR imaging, and endoscopic clearance of sizeable polyps given their potential for development of cancer and intestinal obstruction [104]. Surveillance should be initiated at 8 years of age or earlier if the patient is symptomatic and continued every 3 years if polyps are detected at the index examination. If few or no polyps are found at the initial examination, screening should start again at the age of 18.
Among the disorders predisposing to small-bowel cancer, Lynch syndrome must also be mentioned, due to germline mutations in one of the mismatch repair genes. The lifetime risk of developing small-bowel cancer is estimated to be around 4 %. However, little is known about prevalence and natural history of these tumors and surveillance in this subset of patients is not yet recommended [105]. In a recent prospective series of 35 patients with Lynch syndrome undergoing CE and CTE, CE diagnosed a histologically confirmed cancer in 8.6 % of cases, compared to CTE that missed all but one neoplasia [106]. Despite these promising results, to date the usefulness and cost-effectiveness of small-bowel screening has not been confirmed.
Outcome and Results in the Old and New Era of Endoscopy and Radiology
Familial Adenomatous Polyposis
Prophylactic colectomy has greatly improved life expectancy for FAP patients and disease-related morbidity and mortality causes shifted from CRC to duodenal and ampullary adenocarcinomas and to extraintestinal manifestations such as desmoid tumors [107]. Lifetime risk to develop duodenal adenomatosis (Fig. 13.11) is 100 %, with an increasing severity with aging [93]. The severity of duodenal polyposis is rated according to the classification derived by Spigelman and describes five stages from 0 to IV, based on polyp number, size, histology, and degree of dysplasia [95, 108]. Aging of subjects is a determinant factor for progression of duodenal adenomatosis. On the other hand, technical improvements in endoscopic imaging as well contribute to explaining the progression in Spigelman stages, from low to high stages, that has been recorded over the past years [109]. However, the progressive duodenal and periampullary polyposis with advancing age remains a relatively slow process [109–111], as is the progression to cancer. The lifetime risk of developing duodenal cancer is estimated to be approximately 5 % in most series. The current guidelines recommend surveillance of the duodenum with a side-viewing endoscope, to allow detailed inspection of the papilla, and a forward-viewing instrument, at intervals dictated by the Spigelman stage [98]. It has been demonstrated that the higher the Spigelman stage, the higher the risk of small-bowel adenocarcinomas, with a lifetime risk up to 7–36 % in patients with Spigelman stage III and IV, over follow-up periods of 7–10 years [93, 94].
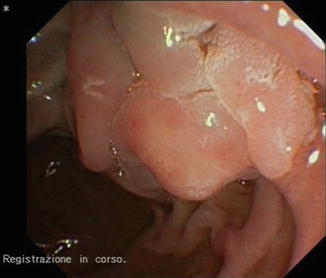

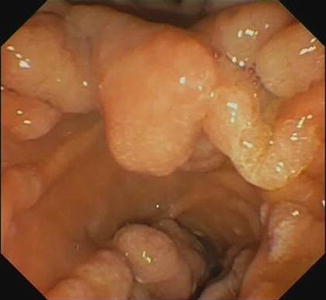
Fig. 13.11
Large duodenal adenomatous polyp in FAP
Endoscopic treatment of duodenal and ampullary adenomas is a crucial aspect of the care of FAP patients. With regard to their endoscopic appearance, small-bowel FAP adenomas appear like nonpolypoid, crackled, whitish plaques, or flat lesions, at times coalescing to produce a carpet-like change of the mucosal surface. Even normal-appearing duodenal mucosa may harbor adenomatous dysplastic tissue. High-definition, high-resolution white light imaging, combined with dye spraying or virtual chromoendoscopy, such as NBI or FICE, may help to better demarcate these lesions and improve detection [112, 113]. The main current therapeutic option is endoscopic snare resection, when polyps are amenable to endoscopic treatment. Often duodenal FAP adenomas are numerous (Fig. 13.12), and not all polyps can be removed. On the basis of expert opinion, patients with few small adenomas may undergo follow-up without endoscopic treatment, whereas patients with Spigelman stage III and IV should undergo endoscopic treatment with removal of significant polyps larger than 1 cm or those with high-grade dysplasia. Intensive surveillance and early treatment may delay the need for major duodenal-pancreatic surgery and its potential detrimental effects as well as lead to a reduction in duodenal cancer mortality [98].