Fig. 6.1
(a) Pillcam SB3 (Given Imaging, Yokneam, Israel); (b) Endocapsule (Olympus Medical Systems Corporation, Tokyo, Japan); (c) Mirocam (IntroMedic Co., Ltd., Seoul, Korea); (d) Capsocam SV1 (Capsovision, Saratoga, USA); (e) OMOM capsule (Jinshan Science and Technology Co., Chongqing, China)
Table 6.1 lists some of the technical differences between the available capsules. The most commonly used capsule is the Pillcam SB series manufactured by Given Imaging (Yokneam, Israel) (Video 6.1). The most recent version, the SB3 (approved by the US Food and Drug Administration in 2013), weighs 3 g and measures 11 mm in diameter and 26 mm in length. It is equipped with a CMOS image sensor, a short focal length lens, four white light-emitting diodes for illumination, and an ultrahigh-frequency radio telemetry transmitter for communication of video data to a portable recorder worn by the patient. The angle of view is 156°, and the minimal detection size is estimated to be 0.07 mm. The capsule features “adaptive frame rate” technology, with video collection rates ranging from two to six frames per second depending on how fast the capsule is traveling. There are two versions of the battery, giving either 8 or 12 h of data collection time. The capsule is used with the RAPID Recorder DR3 and RAPID Sensor Belt SB3 accessories. There is an external real-time image viewer (RAPID Real Time Viewer), which is helpful to determine if the capsule has reached the colon and whether the study can be terminated early. The software program has also undergone incremental improvements. The most recent version (RAPID Reader 8.0) includes an advanced A-mode feature for video compilation, as well as flexible spectral imaging color enhancement (FICE) contouring and a progress indicator, based on time elapsed, linear distance traveled, and capsule motion information, to assist in localizing lesions for therapeutic intervention. There is also a function to describe findings semiquantitatively using the Lewis Score [4]. Figures 6.2 and 6.3 show representative images of small bowel pathology by the Pillcam SB2 and SB3.
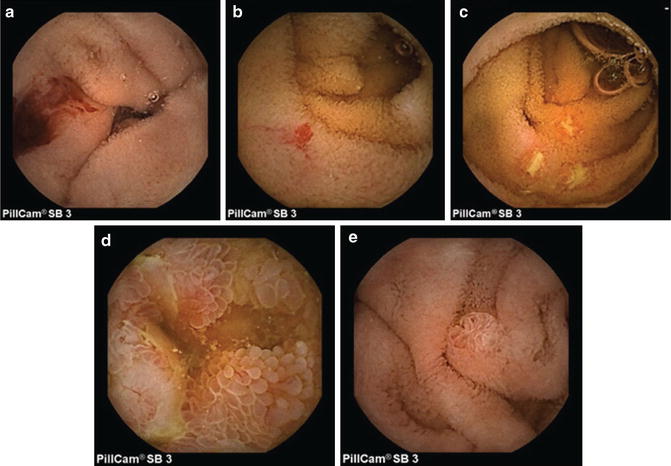
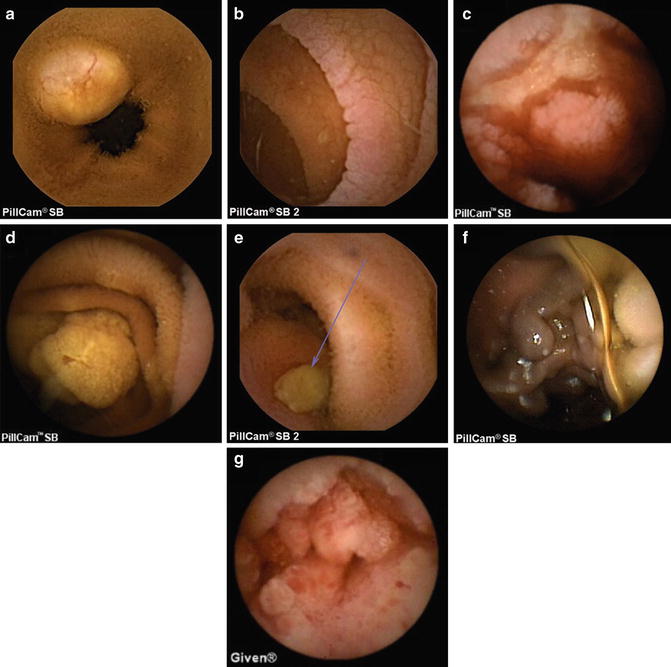
Table 6.1
Current small bowel capsule endoscopes that are commercially available or in development
Capsule | Manufacturer | Dimensions (mm) | Angle of view (°) | Image capture rate (fps) | Battery life (h) | Data transmission technology |
|---|---|---|---|---|---|---|
Pillcam SB3 | Given Imaging | 11 × 26 | 156 | 2–6 | 12 | Radiofrequency |
Endocapsule | Olympus | 11 × 26 | 145 | 2 | 8 | Radiofrequency |
Mirocam | IntroMedic | 10.8 × 24.5 | 170 | 3 | 12 | Electrical field propagation |
Capsocam SV1 | Capsovision | 11 × 31 | 360 | 12–20 | 15 | Capsule retrieval/download |
OMOM JS-ME-II | Jinshan | 13 × 27.9 | 140 | 2 | 8 | Radiofrequency |

Fig. 6.2
Pillcam SB3 capsule images of: (a) bleeding; (b) angioectasia; (c) Crohn’s ileitis with ulcers; (d) edematous villi; (e) normal papilla of Vater

Fig. 6.3
Pillcam SB or SB2 capsule images of (a) carcinoid tumor; (b) celiac sprue; (c) cytomegalovirus enteritis; (d) small bowel polyp in the setting of familial adenomatous polyposis syndrome; (e) gastrointestinal stromal tumor with ulcer; (f) jejunal varices; (g) small bowel adenocarcinoma. Images courtesy of Dr. Michael Chiorean
The Endocapsule EC-S10 (Olympus America, Allentown, Pennsylvania) is almost identical in size to the SB3, with a weight of 3.3 g. This device captures two frames per second, using a supersensitive CCD image sensor with high resolution. In addition, the Endocapsule features automatic brightness adjustment capabilities similar to that used in Olympus endoscopes and has a battery life of 12 h. The accompanying software (Endocapsule Software 10) has red color detection and 3-dimensional tracking functions. A small study on patients with obscure small intestinal bleeding showed reasonably good agreement between slightly older versions of the Pillcam SB and Endocapsule, but did not demonstrate any definite superiority of one capsule over the other [5]. A previous European study also failed to show any significant difference in diagnostic yields between the two [6].
The Mirocam MC1000-W (Intromedic, Seoul, Korea) has recently been FDA approved but is not yet in widespread use in the USA. Small comparative studies have reported equivalent outcomes between the Mirocam and Pillcam SB or Endocapsule in terms of yield and findings [7–10]. An associated model, the MC1000-WM, has limited ability to be steered in real time.
The Capsocam SV1 (Capsovision, Saratoga, California) features 360° panoramic viewing (via four cameras), capturing 20 frames per second (five for each camera) for the first 2 h followed by 12 frames per second for the remainder of the 15-h battery life. It is slightly larger than the other capsules, with dimensions of 11 mm by 31 mm. Its smart motion sense technology activates the cameras only when the capsule is in motion, limiting the number of redundant images and improving battery life. There are 16 white light-emitting diodes powered by an automatic light controller. However, unlike the other capsules, the data are not wirelessly transmitted to a receiver; instead, the capsule must be retrieved after passage from the body and the data downloaded via a direct connection. This may potentially adversely affect patient acceptance. On the other hand, the presence of cardiac pacemakers or implanted defibrillators is not a listed contraindication, and there is no need for the patient to wear an external receiver. Small studies have shown comparable diagnostic yield and image quality between the Capsocam and Pillcam SB, but reading time with the Capsocam was longer [11, 12]. Finally, the OMOM JS-ME-II capsule (Jinshan Science and Technology, Chongqing, China) is slightly larger than the Pillcam SB3, but other technical specifications are similar. An associated version, the OMOM JS-ME-III, is controllable in real time; this function is mainly intended to move the capsule around when examining the stomach. Uncontrolled studies have shown promising diagnostic yields and rates of complete small bowel examination [13, 14]. Currently, neither the Capsocam nor the OMOM capsule are approved by the FDA for use in the USA.
The following comments, unless otherwise specified, apply to the Pillcam SB series, the longest approved and most commonly used capsule endoscope in the USA.
Bowel Preparation and Procedure Protocol
The capsule is swallowed following an 8–12-h fast and, in many cases, some form of bowel preparation. Patients are typically allowed to drink clear liquids 2 h into the study, and to eat solid food 4 h after capsule ingestion. During the 8–12 h of battery life, the capsule passes through the gastrointestinal tract via peristalsis while images are recorded and transmitted wirelessly to an external recorder worn by the patient. The images are formatted into a video file that can be viewed on a computer using specialized software. For patients who are unable to swallow the capsule or suffer from delayed gastric emptying, the capsule can be deployed endoscopically in the duodenum or postoperative anatomy using a specially designed capsule holder fitted onto the tip of a conventional endoscope.
Currently, there is wide variation in the bowel preparation process used in different units. Various types of osmotic or stimulant laxatives, such as magnesium citrate or bisacodyl, and prokinetic agents, such as metoclopramide, may be used to prepare the small bowel for capsule endoscopy. Three meta-analyses have concluded that mucosal visualization was better when the bowel was prepared with sodium phosphate, polyethylene glycol, or erythromycin compared with clear liquid diet alone [15–17]. A 2 L polyethylene glycol preparation has been proven to be equally efficacious to a 4 L preparation in terms of mucosal visualization and capsule completion rate [18]. A score has been developed based upon the proportion of mucosa visualized and quantification of the degree of obscuration by bubbles, debris, or bile [19]. The interobserver agreement of this scale was shown to be good (k = 0.8), although it has yet to be validated prospectively.
Diagnostic Yield for Obscure Bleeding and Anemia
The diagnostic yield (i.e., the percentage of capsule studies demonstrating clinically significant findings) varies considerably depending on indication and patient characteristics. Reported diagnostic yields are 36–92 % for obscure overt gastrointestinal bleeding, 41–63 % for occult bleeding, and 42–66 % for unexplained iron-deficiency anemia [20–33]. The most common finding explaining obscure bleeding is small bowel angioectasia. Serious findings in these studies were particularly common in young patients with unexplained anemia [34]. However, performing capsule endoscopy in patients who had only a positive fecal occult blood test (without anemia or visible bleeding) was not fruitful [35]. Most studies have not been able to report true accuracy data (such as sensitivity or specificity) due to the difficulty of establishing a reliable gold standard for obscure small bowel bleeding.
Several meta-analyses have summarized the data on comparative studies involving capsule endoscopy for small bowel evaluation [36–38]. For obscure bleeding, the diagnostic yield of capsule endoscopy proved superior to that of push enteroscopy (with a yield gain of 30 %), small bowel barium studies (gain of 36 %) [38], and magnetic resonance enteroclysis [39]. In fact, capsule endoscopy was almost as accurate as intraoperative endoscopy [38, 40]. Another meta-analysis concluded that capsule endoscopy had a higher yield than “unidirectional” double-balloon enteroscopy, but was inferior to “bidirectional” double-balloon enteroscopy; that is, when per-oral and per-rectal approaches were both used [41]. Since bleeding lesions (including tumors) can be missed by capsule endoscopy [42], repeat capsule endoscopy can be useful in a significant proportion of patients [43–45].
The clinical impact of capsule endoscopy on patient management is important, because nonspecific, clinically insignificant lesions are often found, even in asymptomatic individuals [46]. Studies have generally shown that capsule endoscopy often positively influences clinical management and outcomes, although some recent studies have raised doubts as to its clinical impact on long-term outcomes [47, 48]. For “positive” capsule studies for obscure bleeding, 44–82 % lead to specific therapeutic interventions or changes in management, and 63–83 % are associated with cessation of bleeding [21, 24–26, 49, 50]. Some studies have reported that negative capsule studies can predict a lower risk of rebleeding [51–54], although data have been discordant in other studies [55–57]. For iron-deficiency anemia, the medium-term impact has proven modest despite relatively high diagnostic yields [28, 58, 59]. In the acute setting, immediate capsule endoscopy has been shown to be more useful than angiography [60].
Diagnostic Yield for Crohn’s Disease and Other Non-bleeding Indications
In up to one-third of patients with Crohn’s disease, inflammation is confined to the small intestine, and beyond the reach of the push enteroscope or colonoscope [61, 62]. Barium small bowel follow-through examinations have limited ability to detect mild mucosal inflammation in early Crohn’s disease [37, 63], and have been supplanted by computerized tomographic enterography (CTE) and magnetic resonance enterography (MRE), which can also demonstrate extraluminal disease such as abscesses or fistulae. However, capsule endoscopy is probably superior to CTE or MRE for detecting superficial mucosal ulcerations. The diagnostic yield of capsule endoscopy, CTE, and MRE were compared in a meta-analysis of 12 trials [64]. Capsule endoscopy enjoyed significant incremental yield over that of ileocolonoscopy (22 % gain) and CTE (47 %). A subsequent study on suspected or newly diagnosed Crohn’s disease compared capsule endoscopy against CTE and MRE [65]. Patients underwent ileocolonoscopy, CTE, or MRE, followed by capsule endoscopy. The sensitivity and specificity for the diagnosis of terminal ileitis were 100 % and 91 % for capsule endoscopy, compared with 81 % and 86 % for MRE, and 76 % and 85 % for CTE.
In patients with symptoms suspicious for Crohn’s disease, the combination of capsule endoscopy and ileocolonoscopy identified 97 % of all small bowel inflammatory lesions, while small bowel follow-through and ileocolonoscopy detected only 57 %. Of the patients ultimately found to have small bowel Crohn’s disease, 55 % were diagnosed by capsule endoscopy alone [61]. It should, however, be noted that small bowel erosions seen on capsule endoscopy may be nonspecific or due to the use of nonsteroidal anti-inflammatory drugs (NSAIDs); furthermore, there have been well-documented cases of small bowel ulcers being identified even in “normal” individuals [46, 66].
Capsule endoscopy can also be helpful in monitoring the extent and severity of inflammation in patients with established Crohn’s disease [67]. In persistently symptomatic patients, capsule endoscopy identified active inflammation in 82 % of patients, compared with only 49 % detected by ileocolonoscopy [68]. In another study, 56 % of subjects were noted on capsule endoscopy to have jejunal ulcerations not seen on CTE [69, 70]. Capsule endoscopy is also useful for documenting mucosal healing after treatment [1–3, 67, 71]. In a prospective study, capsule endoscopy performed before and after treatment demonstrated a significant reduction in the number of small bowel ulcers, and mucosal healing correlated with other measures such as the Crohn’s Disease Activity Index, Inflammatory Bowel Disease Questionnaire, and C reactive protein values [72]. Lastly, capsule endoscopy can be used to screen for anastomotic recurrence of Crohn’s disease after surgical therapy [73, 74].
In 4–10 % of patients with inflammatory bowel disease involving the colon, distinguishing ulcerative colitis from Crohn’s disease is not possible with just ileocolonoscopy and imaging [2, 75]. In some situations, establishing this distinction has important implications for medical and particularly surgical treatment. By providing direct visualization of the entire small bowel, capsule endoscopy can often clarify the diagnosis in patients initially presenting with indeterminate colitis. Studies have shown that capsule endoscopy altered the diagnosis in 29–40 % of such patients [62, 76–78].
Capsule Endoscopy Scoring Systems for Crohn’s Findings
Currently, there are two validated scoring systems available to describe the extent and severity of Crohn’s disease seen on capsule endoscopy. The Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI) is based on the severity of inflammation, extent of disease, and the presence or absence of strictures. The score ranges between 0 and 36, with higher numbers representing more severe disease [87, 88]. The Lewis Index is based on villous edema, mucosal ulceration, and luminal stenosis [4]. A score of <135 is normal, while a score of ≥790 denotes moderate to severe inflammation [89]. However, studies have suggested that correlation is poor between the CECDAI and fecal calprotectin, while the Lewis score only correlates with low calprotectin levels [90, 91]. Correlation is mediocre between the CECDAI and C-reactive protein levels [92].
Comparison of Capsule Endoscopy and Deep Enteroscopy
Double- or single-balloon-assisted enteroscopy, also termed deep enteroscopy, is an alternative means for evaluating the entire small bowel that has become available in the last decade. This method is somewhat limited by the special training required and the frequent need for extended procedure times and anesthesia support, but sometimes can detect lesions missed by capsule endoscopy [93]. A meta-analysis reported diagnostic yields of 57 % with capsule endoscopy and 60 % with deep enteroscopy. Stratified analysis looking at vascular lesions, inflammatory changes, and small bowel polyps/tumors all showed comparable yields between capsule endoscopy and deep enteroscopy [94]. More recent meta-analyses also concluded that the two modalities were comparable and complementary [95, 96], although deep enteroscopy had an increased yield when performed after a positive capsule endoscopy. Similarly, intraoperative enteroscopy also had an increased yield if performed after a positive capsule endoscopy [97]. In general, capsule endoscopy would be recommended as the initial test of choice in the evaluation of small bowel disease, and can be followed up by deep enteroscopy if indicated. Intraoperative enteroscopy is the test of last resort in extremely recalcitrant obscure bleeding patients.
Contraindications and Complications
Contraindications to capsule endoscopy are few and the complication rate is low [98, 99]. The most important potential complication is capsule impaction in the small bowel. According to guidelines, capsule impaction is defined as retention of the capsule in the small bowel for longer than 2 weeks [100]. Capsule impaction should be distinguished from type 1 regional transit abnormality, in which the capsule stays at the same point for more than 60 min (but less than 2 weeks) with no abnormality visible on the capsule video image [101, 102], or type 2 regional transit abnormality (previously termed transient capsule retention), in which the capsule stays at the same point for >60 min (but less than 2 weeks) with a visible abnormality such as a stricture [103]. Regional transit abnormalities are fairly common, with reported incidence rates of 5.7–13.3 % [104]. Capsule impaction is much less common, with occurrence rates influenced by the indication and patient characteristics. A systematic review reported capsule retention rates of 1.2 % for obscure bleeding, 2.6 % for Crohn’s disease, and 2.1 % for small bowel tumors [105]. There have been no reported cases of capsule impaction occurring in a normal small intestine [46]. Capsule impaction usually occurs at sites of structural abnormality in the small bowel, such as ulcers, masses, strictures, or surgical anastomoses [106].
Most cases of capsule impaction are asymptomatic, even when the capsule remains impacted for very long periods (as long as 2.5 years) [104, 107]. There have been only a few reported cases of symptomatic capsule retention, including capsule entrapment in a Meckel’s diverticulum [108], acute obstruction in a Crohn’s disease patient [109], capsule impaction in the appendix leading to appendicitis [110], and intestinal perforation [111, 112]. Once diagnosed, options to treat capsule retention include endoscopic retrieval or, in the majority of cases, surgery. Capsule retention helps to localize the site of the pathology, allowing the surgeon to target his or her intervention and potentially remove the offending lesion at the same time as the impacted capsule [100].
Radiographic studies can identify possible obstructive intestinal lesions, but cannot reliably predict capsule impaction [98, 107]. The capsule has been known to pass normally even in patients with radiographically evident strictures [113]. Nevertheless, in some situations, small bowel follow-through, and more recently CTE or MRE, may be helpful if performed before capsule endoscopy in patients at increased risk for obstruction.
Another predictive tool is the Pillcam Patency Capsule (Given Imaging, Yokneam, Israel), which is a radio-opaque, self-dissolving capsule made of lactose and barium with two side timer plugs with exposed windows. It is the same size and shape as the Pillcam SB3 and contains a radiofrequency identification tag that allows it to be detected by an external scanning device before it disintegrates 30 h after ingestion [106, 113–115]. In patients with known or suspected small bowel strictures, the patency capsule had a 91 % negative predictive value for capsule retention [116]. In one study, in which only those patients successfully passing the patency capsule were selected for capsule endoscopy, the capsule retention rate was zero [114]. Overall, the patency capsule seems to be a useful tool for assessing certain high-risk patients prior to capsule endoscopy [117]. Rare complications from the patency capsule, such as transient intestinal occlusion and abdominal discomfort, have been reported [114, 118].
The radiofrequency waves used by the capsule endoscope to transmit data to the recorder can pose a theoretical risk of interference with implanted electronic devices. Based on this concern, the FDA and the manufacturer, Given Imaging, list the presence of cardiac pacemakers or defibrillators as a contraindication to capsule endoscopy. However, data from a number of studies have demonstrated that capsule endoscopy does not result in any cardiac arrhythmias or alteration of implanted electronic device function [119–123]. A brief lapse in capsule image acquisition (less than 2 min) was noted in only two patients, who remained asymptomatic and had no adverse events. Such data suggest that capsule endoscopy is probably safe to perform in patients with implanted electronic devices.
Current Limitations
Capsule endoscopy, in its present form, still suffers from several limitations. Some problems are logistical, such as difficulties with insurance coverage, reimbursement for capsule study interpretation, and training and quality control. However, capsule endoscopy has inherent technical limitations as well. One of the most important drawbacks is its inability to precisely localize detected lesions in order to target further therapy. The temporal relationship of visualized lesions with the pyloric channel and ileocecal valve gives the examiner an approximate idea of where the lesion is, but this can be misleading because the speed of capsule movement along the small bowel is not constant. The capsule also lacks the ability to mark the location of detected lesions (such as with a tattoo), a function that would be helpful for subsequent endoscopic or surgical therapy. Furthermore, delayed gastric emptying and slow small bowel transit can lead to exhaustion of the battery before the cecum is reached. In some older studies, this occurred in up to 20 % of capsules deployed, although longer battery life and adaptive frame collection technology in the Pillcam SB3 may alleviate this problem in the future [124]. Reducing the frame rate in OMOM capsules has been shown to improve complete small bowel examination rate without adversely affecting diagnostic yield [125, 126].
Capsule endoscopy visualization can be impaired by the presence of bubbles, food materials, suboptimal lighting, or resolution problems. The capsule examiner can repeatedly scrutinize video frames of interest, but cannot retroactively obtain better images; in contrast, during standard endoscopy the examiner can scrutinize the area of interest repeatedly in real time, maneuver the endoscope to adjust perspective, angle or distance, and perform flushing, suctioning, or air insufflation as needed. Because the movement of the capsule through the intestines is entirely passive, there is no way to control capsule movement in real time to improve the image quality in areas of interest. The random motion of the capsule means that in some frames only part of the 360° circumference of the small bowel is visible. Capsules with an extremely wide angle of view using multiple cameras, such as the Capsocam, may address this issue in the future. Finally, current capsules are purely diagnostic and have no capability to obtain biopsies or intervene therapeutically.
Conclusion
Future Directions
Since its advent, most of the advances in capsule technology have been evolutionary in nature. Further improvements in resolution, brightness control, and angle and depth of view will undoubtedly occur, but their impact will be limited. On the other hand, breakthroughs in lesion localization and therapeutic intervention, when they occur, will be revolutionary [124]. Some of the possible future roles for capsule endoscopy include the performance of biopsies, medication injection, tattooing, capsule ultrasonography, and argon plasma coagulation. Most of these capabilities require real-time imaging and the ability to control the capsule. Already there are capsules in development with limited steering capabilities, such as some versions of the Capsocam and OMOM capsule. The most recent versions of the Pillcam SB and Endocapsule both feature a limited real-time imaging function. In the emergency room, capsule endoscopy can be used to rule out upper gastrointestinal bleeding in real time in order to assist triage. Preliminary studies have shown that capsule endoscopy in the acute setting is feasible and useful [127–130].
In the future, true real-time control capabilities may become technically feasible, but there are still some logistical challenges. Since the small intestinal transit time can range from 30 min to 12 h, it would not be practical to perform real-time imaging for all capsule studies. A more realistic approach would be for all patients to first undergo diagnostic capsule endoscopy with retroactive examination of the video recording, similar to what is currently done. If a lesion is potentially amenable to capsule treatment, then the patient can be referred for a more involved therapeutic capsule procedure performed with real-time control. Continuous advances in capsule technology are taking place, with capsule endoscopes for evaluation of the esophagus and colon making their appearance in the commercial market in the last few years. Small bowel capsule endoscopy has already been shown to be safe and cost effective [131], and the learning curve for reading capsule endoscopy is relatively modest [132]. It is likely that the scope of capsule endoscopy will continue to expand. We look forward to a day when we can finally examine the darkest recesses of the small bowel with the same accuracy and ease that we currently enjoy for the stomach and colon.
References
1.
Swain P, Adler D, Enns R. Capsule endoscopy in obscure intestinal bleeding. Endoscopy. 2005;37(7):655–9.PubMed
2.
Bourreille A, Ignjatovic A, Aabakken L, Loftus Jr EV, Eliakim R, Pennazio M, et al. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: an international OMED-ECCO consensus. Endoscopy. 2009;41(7):618–37.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








