The proportion of visualised mucosa
Score 3 (%)
≥75
Score 2 (%)
50–75
Score 1 (%)
25–50
Score 0 (%)
<25
The degree of bubbles, debris and bile
Score 3
<5 %, not obscured
Score 2
5–25 %, mildly obscured
Score 1
25–50 %, moderately obscured
Score 0
≥50 %, severely obscured
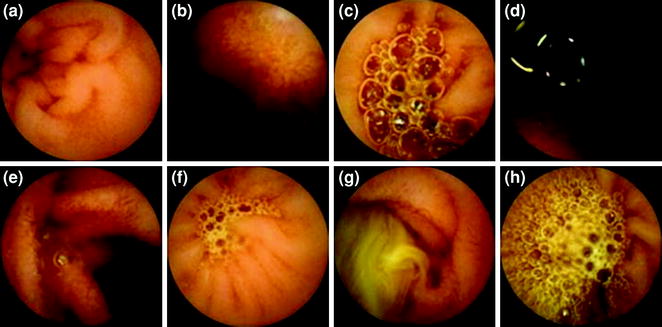
Fig. 3.1
Images of scores according to the proportion of the visualised mucosa (a–d) and the degree of obscuration (e–h). a Score 3; b Score 2; c Score 1; d Score 0; e Score 3; f Score 2; g Score 1; h Score 0
3.1.4 Post-capsule Ingestion Preparation
Some accommodation needs to be made for differences between sensors and receivers of different manufacturers with regards to pre- and post-preparation. There are currently 5 commercially available capsules; MiroCam by Intromedic, PillCam by Given Imaging , EndoCapsule by Olympus , OMOM capsule by Jianshan Science and Technology Ltd and CapsoCam by Capsovision.
Most capsule manufacturers advocate a complete fast (no fluid intake at all) for at least 4 h before and 2 h after ingestion of the capsule in addition to oral bowel preparation ; these manufacturers rely on radiofrequency transmission. The MiroCam system utilises human body communication for transmission of images from the capsule to the receiver, and the manufacturers do not advocate the need for a water-free fast period, but encourage sipping on water hourly after ingestion as it potentially may enhance image transmission.
3.1.5 Practical Issues Related to Delayed Gastric Emptying/Small Bowel Transit
Apart from oral preparation, it is worthwhile considering the characteristic of the patient particularly with regards to preventing an incomplete examination of the small bowel and improving mucosal visualisation. Iron supplementation and loperamide containing medications should be stopped 5–7 days before the procedure; the former obscuring views from unabsorbed iron and the latter having an impact in decreasing intestinal motility.
Gastric and colonic transit time s vary greatly in comparison with small bowel transit between individuals. It is known that delayed gastric emptying is mainly responsible for incomplete examinations [23, 24]. Hence, scenarios that may affect gastric transit should be noted by capsule operators. Gastric retention and emptying were studied extensively, even before the advent of small bowel capsule, and it seems that there are numerous factors that can affect gastric emptying [25]. Anecdotally and with reasonable evidence, there are a number of conditions and drugs that are known to effect gastric and possibly small bowel transit. Table 3.2 lists the most common of these. If possible, all contributing drugs should be stopped a few days before the examination.
3.1.6 Sample Regimen
The following lists some potential regimen s (see post-capsule ingestion preparation in regards to clear water period). All regimens cater for the capsule ingestion occurring in the morning (most practical). Essential medications can be taken as required with sips of water.
1.
Breakfast the day before, then clear fluids only from 12:00. Two litres of PEG solution taken between 16:00 and 18:00. Complete fast from midnight.
2.
Normal lunch the day before, then clear fluids only from 15:00. Ingestion of 1 L of PEG solution between 30 min and 2 h post-ingestion of capsule
For patients who have had a failed procedure as a consequence of poor cleanliness, consider restricting to a full 24 h of clear liquids only (with possible low residue dietary restriction 48 h before), doubling the volume of prep and adding in 160 mg simethicone at the time of ingestion.
For patients who have had a failed procedure as a consequence of not reaching the caecum in time, consider administration of 10–20 mg metoclopramide 30 min before ingestion or around the time of capsule ingestion if by IM or IV administration.
3.1.7 Conclusion
Bowel preparation is essential to provide adequate imaging for small bowel capsule endoscopy . Currently, preparation involves fasting, oral cleanser (usually PEG) and avoiding drugs that would obscure or delay gastrointestinal transit. Promotility drugs are used if required for individual patients. Further research is required with larger studies to devise better tolerated regimen s.
3.2 Obscure Gastrointestinal Bleeding
Abstract
The evaluation of patients with obscure gastrointestinal bleeding (OGIB) has been suboptimal for a long time due to the limited ability to image the small bowel. Over the past 10 years, significant improvements have been made in small bowel imaging techniques. Particularly, since the introduction of capsule endoscopy (CE), the diagnostic approach to OGIB has improved significantly. CE is an extremely well-tolerated examination that allows the evaluation of the entire small bowel mucosa, providing high-quality images and identifying even tiny, flat mucosal changes (i.e. vascular malformation s, which represent the most frequent small bowel lesion found in patients with OGIB). Many studies have shown that the diagnostic yield of CE in OGIB patients, which is 50–60 %, is significantly superior to that of most conventional techniques for the study of the small bowel and similar to highly invasive procedures such device-assisted enteroscopy (DAE). Therefore, nowadays, CE is considered the examination of choice in patients with OGIB, after negative gastroscopy and colonoscopy. Its diagnostic yield is especially high in patients with overt bleeding, or when the procedure is performed close to the last episode of acute bleeding, as well as in patients with severe anaemia or with high transfusion requirement. When integrated in a global patient care plan, CE is helpful in achieving effective decision-making concerning subsequent investigations and treatments. Future multicentre prospective studies with standardised surveillance and/or treatment protocol, with long follow-up, are warranted in order to truly estimate the long-term impact of CE in patients with OGIB.
Keywords
Obscure gastrointestinal bleeding · Iron-deficiency anaemia · Capsule endoscopy · Device-assisted enteroscopy · Outcome s.
Obscure gastrointestinal bleeding (OGIB) is defined as gastrointestinal (GI) bleeding of unknown origin that persists or recurs following a negative initial endoscopic evaluation (bidirectional gastrointestinal (GI) endoscopy). OGIB is classified as either overt OGIB (which manifests as recurrent melena or haematochezia) or occult OGIB, which presents by recurrent or persistent iron-deficiency anaemia (IDA) [27].
Although OGIB represents only a small proportion (about 5 %) [28] of GI bleeding (Fig. 3.2), it can pose significant diagnostic and management challenges. One of the main reasons is that the small bowel is often the site of bleeding [29, 30]. Indeed, identification and localisation of the bleeding source can be difficult, requiring utilisation of a significant amount of healthcare resources, which leads to reduced cost-effectiveness [31]. Patients with OGIB often require numerous blood transfusions and repeated hospital admissions involving multiple diagnostic procedures. Therefore, the advent of capsule endoscopy (CE) was a major forward leap, leading to a complete redesign of diagnostic algorithms for patients with OGIB [32–36].


Fig. 3.2
Causes of gastrointestinal bleeding through the GI tract
The success story of CE lies in its technical characteristics. Hence, its unique ability to provide high-resolution images of the entire (in the majority of cases) [37, 38] small bowel mucosa can account for its superiority over other ‘conventional’ endoscopic modalities. To date, several studies have shown that, in terms of diagnostic yield (DY), CE is superior, when compared to push enteroscopy (PE) [39–42] (which usually enables the exploration of only 100–150 cm of small intestine), or to DAE [43, 44], if the latter is carried out with a single approach (oral or anal). Conversely, there is a substantial equivalence between CE and DAE when the latter (combining oral and anal approach) assess the entire small bowel [44, 45].
Furthermore, as it was rather expected in patients with OGIB, CE demonstrated better diagnostic performance when compared to classical radiologic modalities, e.g. small bowel follow through. Although most radiological methods provide—just like CE—views of the entire small bowel, they are ‘betrayed’ by their low spatial resolution in cases of small, superficial lesions such as vascular malformation s, erosions or ulcers [46, 47].
3.2.1 Findings at Capsule Endoscopy in Patients with OGIB
Essentially, the spectrum of lesions responsible for small bowel bleeding is similar to that seen in other GI tract segments. It includes vascular and inflammatory lesions, as well as polyps/neoplasms. The prevalence of different small bowel findings in patients with OGIB and positive CE is reported in Fig. 3.3.


Fig. 3.3
Patients with OGIB and positive CE: spectrum of findings
3.2.1.1 Vascular Lesions
Vascular lesions are the most common causes of bleeding in the small intestine, accounting for about 50–60 % of all small bowel findings [48]. Amongst vascular lesions, angioectasias represent the most frequent small bowel finding in adult patients living in Western countries. Angioectasias (also called arterio-venous malformations–AVM), appear as small, superficial red spots with well-demarcated edges (Fig. 3.4). They are characterised by dilated, distorted blood vessels.


Fig. 3.4
AVMs identified by CE; a and c are usually classified as P2 lesions whereas b as a P1 lesion
A less common vascular lesion in the small bowel is Dieulafoy’s lesion, which consists of an abnormal, submucosal ‘calibre-persistent artery’ that typically protrudes through a minute 2–5-mm mucosal defect. In most cases, diagnosing a Dieulafoy’s lesion is challenging, as by definition, it relies on visualising acute arterial bleeding or a protruding vessel with or without active bleeding during endoscopy. In the absence of these findings, a small mucosal ulceration overlying an artery can be easily overlooked.
Small bowel varices, appearing at CE as tortuous, bluish nodular structures, are seen in the setting of portal hypertension due to chronic liver disease, portal vein thrombosis and/or hepatic vein thrombosis. Ectopic varices are usually located within the proximal small bowel (duodenum and jejunum) and are overall less likely to rupture, compared with oesophageal varices. In portal hypertensive enteropathy (PHE) (Fig. 3.5) small bowel varices may be associated with angioectasias , which are usually minute and petechial in appearance [49].


Fig. 3.5
Portal hypertensive enteropathy with marked and diffuse folds and villous oedema
Venous and phlebectasis, (Fig. 3.6) appearing as bluish, flat or slightly elevated spots, are other forms as vascular lesions. This finding is extremely common in the small bowel and usually of no clinical significance; nevertheless, when phlebectasis are multiple, elevated, nodular, large in diameter and/or associated with skin or mucosal venous blebs (cavernous skin haemangioma), can be a manifestation of the blue rubber bleb nevus syndrome or bean syndrome, a rare clinical condition (200 cases are reported in the literature) [50], which is often complicated by profuse GI haemorrhage (which is the main cause of mortality in these patients) [51] (Fig. 3.7).



Fig. 3.6
Phlebectasis

Fig. 3.7
Giant cavernous haemangioma with active bleeding
3.2.1.2 Inflammatory Lesions
Although the appearance of inflammatory small bowel lesions is similar to those encountered in other GI segments, they do not appear as depressed at CE, because of the lack of insufflation. Conversely, the fibrin covering the central part of ulcers is often elevated, when compared with the surrounding mucosa (Fig. 3.8).


Fig. 3.8
Inflammatory lesions identified in the small bowel of four patients taking NSAIDs; a erosions; b small ulcers covered by fibrin; c, d circumferential ulcers causing narrowing of the lumen
Small bowel inflammatory changes are seen in several conditions, such as inflammatory bowel disease (IBD), (Fig. 3.9) (previously undiagnosed Crohn’s disease is found incidentally in approximately 6 % of patients undergoing CE to evaluate OGIB) [48], small bowel lymphomas, (Fig. 3.10) chronic mesenteric ischaemia, complicated coeliac disease , (Fig. 3.11) tuberculosis , Behcet’s disease, eosinophilic enteritis, and conditions as chronic non-specific multiple ulcers of the small intestine (CNSU) or cryptogenic multifocal ulcerous stenosing enteritis (CMUSE). Noteworthy, a number of different drugs, such as potassium, chemotherapeutic agents, 6-mercaptopurine, and mainly acetylsalicylic acid and non-steroidal anti-inflammatory drugs (NSAIDs ), can cause small bowel ulcerations and bleeding.




Fig. 3.9
Cobblestone and linear ulcerations in the terminal ileum of a patient with Crohn’s disease

Fig. 3.10
Small bowel lymphoma appearing at CE as a polypoid lesion by whitish irregular mucosa

Fig. 3.11
Proximal small bowel of a patient with refractory coeliac disease ; presence of scalloping of folds, mucosal fissures and erosions
NSAID-induced enteropathy may occur with both cyclooxygenase-1 and cyclooxygenase-2 inhibitors agents [52]. Unfortunately, almost all of the inflammatory lesions in the small bowel have a non-specific appearance; therefore, although CE can be helpful in identifying the source of bleeding, when inflammatory changes are identified a precise diagnosis can seldom be made on the ground of endoscopic findings alone (Fig. 3.8). At present time, this represents one of the main limitations of CE in the setting of the OGIB. Imaging enhancement tools such as virtual chromoendoscopy and/or 3D imaging could potentially have a differential in the classification of such lesions [53, 54].
3.2.1.3 Small Bowel Neoplasms/Small Bowel Polyps
Small bowel tumours are discovered in 2–10 % of patients undergoing CE because of OGIB [55, 56], particularly in young patients [57]. Benign and malignant primary tumours, as well as metastatic tumours (i.e. melanoma or breast cancer) [58–60], can be found throughout the small intestine. The endoscopic appearance of small bowel tumours varies according to their size, location and site of origin. Tumours originating from the mucosa [i.e. adenocarcinoma s (Fig. 3.12)] often appear as ulcerated masses or large polyps (Fig. 3.13), whereas those originating from the submucosal layer [i.e. neuroendocrine tumours (NET) or gastrointestinal stromal tumours (GIST)] often appear as protruding nodules, covered—at the early stages—by normal small bowel mucosa or later in the course of the disease with superficial erosions or ulcers. The distinction between small bowel submucosal tumours and innocent bulging, due to active peristalsis, is one of the challenging tasks in reviewing CE (Fig. 3.14). A bulge is defined as a round and smooth, large base protrusion in the lumen having an ill-defined edge on the surrounding mucosa; it can be a prominent normal fold or the result of intestinal loop angulation and stiffness, and sometimes, it can be virtually indistinguishable from a small submucosal tumour. Some visual clues may help distinguishing masses from bulges (i.e. changes in mucosal characteristics, presence of bridging folds, of transit abnormalities, of repetitive images, and of synchronous lesions). Some authors [61, 62] have recently proposed, on the ground of these visual clues, a scoring system to distinguish masses from bulges. Unfortunately, none of them has been externally validated yet. Moreover, in everyday clinical practice, the aforementioned indicators are often absent.




Fig. 3.12
Small bowel adenocarcinoma actively bleeding

Fig. 3.13
Large, eroded, small bowel polyp in patient with Peutz–Jeghers syndrome

Fig. 3.14
a Innocent small bowel bulging due to the active peristalsis; b small bowel GIST appearing at CE as a round nodule, covered by slightly congested mucosa (green arrows)
Additionally, several studies [63–65] reported patients with negative CE in which further examinations showed small bowel tumours (false negative CE). Lewis et al. [64], analysing data from an industry-maintained trial database, found that CE was negative in about 1.5 % of patients with small bowel tumours. They estimated that the miss rate of CE in neoplastic diseases can reach 18.9 %. Although this percentage is lower than that reported for other diagnostic modalities (63.2 %), it is still alarming, especially considering the clinical relevance of these missing findings. In addition, recent reports showed a relatively low sensitivity of CE, when compared with computed-tomography (CT) enterography in this setting [66]. Therefore, in patients with clinical suspicion of small bowel tumour, CT enterography should precede CE.
3.2.1.4 Other Small Bowel Lesions
Small bowel diverticulae are frequently overlooked in CE due to lack of insufflation [67] (Fig. 3.15). The prevalence of small bowel diverticulosis is approximately 0.1–2 % of the population. Furthermore, Meckel’s diverticulum is the commonest cause of small bowel bleeding in patients under the age of 25. It is estimated that fewer than 5 % of subjects with jejunal diverticulae actually bleed from them. Moreover, even when discovered by CE, evidence of active bleeding is necessary to conclude that a diverticulum is the site of blood loss.


Fig. 3.15
Ileal diverticulum without stigmata of active or recent bleeding
Another finding, which is identified in about 8–10 % of OGIB patients with positive CE, is the presence of fresh blood/active bleeding in the lumen (Fig. 3.16). Although by definition not a lesion, it is often considered as a positive finding when calculating the DY of CE. Indeed, fresh blood in the bowel lumen confirms the bleeding site in the small bowel, provides useful lesion localisation information, and therefore, it can guide further diagnostic/therapeutic procedures, such as DAE [68].


Fig. 3.16
Red blood in the lumen of the small bowel that covers the mucosa, thus preventing the identification of the actively bleeding lesion
3.2.2 Diagnostic Accuracy of Capsule Endoscopy in Patients with OGIB
Large studies identify a definite bleeding source in 50–60 % of patients [37, 38, 69–71]. The DY is usually calculated taking into account only lesion with high bleeding potential; in fact, SB lesions identified at capsule endoscopy are usually divided into three subgroups: (1) highly relevant lesions (P2) such as angioectasia, large ulcerations, tumours or varices; (2) uncertain relevance lesions (P1) such as red spots, small isolated erosions; (3) low relevance lesions (P0) such as visible submucosal veins, non-bleeding diverticula , nodules without mucosal break [72].
In studies concerning CE, the DY is the parameter conventionally used to estimate the diagnostic ability of this examination. That is mostly because, in this setting, it is very difficult to calculate the most common clinical efficacy parameters such as sensitivity , specificity and diagnostic accuracy , as it lacks a true reference standard to which it could be compared with. Actually, in the study of the small intestine, a reference standard should allow to explore the entire small bowel, recognising the presence of even small lesions and obtaining histological confirmation, when necessary. These goals, at the present time, can be achieved only through intra-operative enteroscopy (IOE) or bidirectional DAE. The first requires of course surgical intervention (associated with mortality and morbidity), whereas the latter is logistically feasible only in a small proportion of the patients [73].
A recent study that compared CE with IOE found that the sensitivity , specificity, positive and negative predictive values of CE were 95, 75, 95 and 86 %, respectively. In this study, the gold standard, i.e. IOE, had 100 % DY for ongoing bleeding, but only 70.8 % for previous overt OGIB and less so (50 %) for occult OGIB [74]. These data confirmed results from an earlier retrospective study [48] in which CE was compared with a composite reference standard, taking into account both further diagnostic examinations and the follow-up. Moreover, in a more recent paper, Tenembaum et al. [67] compared CE with DAE combining oral and anal approach, according to the type of the lesion identified. These authors found that although CE and DAE yielded similar results, CE had an excellent accuracy profile for masses/tumours (sensitivity and specificity : 100 %), an intermediate performance for vascular and inflammatory lesions (sensitivity: 58 and 50 % respectively; specificity: 93 and 98 % respectively) and a disappointing accuracy for small bowel diverticulae (sensitivity : 9 %; specificity : 100 %).
On the basis of solid scientific evidence [69, 70, 75], most national and international gastrointestinal scientific societies have issued updated practice guidelines for the diagnosis and management of patients with OGIB, recommending CE as the first-line tool to evaluate the small bowel, after negative bidirectional endoscopy, in the clinical context of OGIB [32–36].
A key factor, which has been demonstrated in several studies, to significantly affect the yield of CE is the timing of the procedure. In fact, several studies [48, 76, 77], mostly focused on patients with overt OGIB, reported a significantly higher DY (75–90 %) in patients undergoing CE in the early stage of their diagnostic work up. Yamada et al. [77] recently showed an inverse linear correlation between the time elapsed from the last episode of overt bleeding and CE.
Unfortunately, less is known about the exact timing for CE in patients with obscure–occult GI bleeding; in these patients, it is often impossible to establish, in a reliable fashion, the length of the clinical history of anaemia; therefore, a more conservative approach can be generally suggested [78]. Recent guidelines [32, 79] suggest first to exclude other causes of persistent/recurrent iron-deficiency anaemia (i.e. malabsorption, menstrual loss) and then an appropriate empiric therapy with iron supplementation (i.e. ferrous sulphate 200 mg twice daily, for 1–3 months) could reasonably precede CE. Another factor, which has been found to have a relevant impact on the yield of CE, is the severity of bleeding. Patients with OGIB and low haemoglobin levels and/or high transfusion requirement are more likely to have small bowel lesions and deserve a small bowel evaluation by means of CE [80, 81].
It should not be underestimated that patients referred for CE can harbour lesions within the reach of standard endoscopes; nevertheless, in a cost-effective analysis as that performed by Vlachogiannakos et al. [30], their incidence is too low [30, 82] to justify a standard policy of second-look endoscopy before CE. Nevertheless, further studies are needed to identify subgroups of patients at increased risk to harbour lesions within the reach of a conventional endoscope in which repeating gastroscopy and/or colonoscopy would be cost-effective. Therefore, at present time, repeating conventional endoscopy before CE has to be decided on an individual basis, taking into account the reason for referral (occult vs. overt OGIB), but also the quality/timing of previous procedures, ongoing/previous therapies and comorbidities.
It is also important to emphasise that, after negative CE, when patients have ongoing or recurrent evidence of bleeding or when clinical suspicion of small bowel pathology is sufficiently high, further investigation with small bowel (total) enteroscopy or cross-sectional imaging is clearly warranted [83].
3.2.3 Impact on Long-term Outcomes
Although several [48, 71, 75] studies have assessed the yield of CE in OGIB, the exact significance of the lesions identified and their impact on clinical outcome has not been adequately examined. Unfortunately, the majority of studies on CE in OGIB are focused on potential changes in management, rather than on evaluating long-term outcomes. The studies assessing a change in clinical decision-making after CE showed that about two-thirds of patients with OGIB received specific therapeutic interventions or changes in management based on a finding from CE [84, 85].
Some studies [86, 87] reported that a negative CE in patients with OGIB is associated with a low rate of recurrent bleeding; therefore, it is reasonable to take an expectant approach with these patients, thus avoiding the need for unnecessary additional investigations, but keeping these patients under scheduled surveillance.
As far as the long-term outcome is concerned, the majority of studies evaluating the long-term (2–3 years) outcomes in patients undergoing CE report a favourable outcome in most of patients (about 60 %) managed on the ground of capsule endoscopy results [88, 89]. However, recently published studies [90, 91] are casting some doubts on the real impact on long-term outcome , despite a high diagnostic yield of CE. Several factors can probably explain the differences observed in different studies: there are differences in the population characteristics (i.e. medications, type of bleeding, timing of CE, etc.), there are no standardised approaches for patient management after CE and the different policies adopted at different medical centres can introduce relevant bias.
Therefore, although it is well known that CE has the capability to diagnose small bowel lesions and to drive further management [92–96], in the next few years, large multicentre studies with standardised surveillance and/or treatment protocol, with long follow-up, are warranted in order to truly estimate the long-term impact of CE in patients with OGIB.
Conflict of interest statement:
Marco Pennazio is a member of the speaker’s board of Given Imaging Inc. (Yoqneam, Israel). Anastasios Koulaouzidis has received research Grant from Given® Imaging Ltd., Germany (ESGE-Given® Imaging Research Grant 2011) and material support for capsule endoscopy research from SYNMED©, both unrelated to the present work. Emanuele Rondonotti has no conflict of interest.
3.3 The Use of Capsule Endoscopy for Small Bowel Crohn’s Disease
Abstract
The diagnosis of small bowel Crohn’s disease (SBCD) can often be a diagnostic challenge due to varied symptomatology, absence of raised inflammatory markers and non-specific radiological and endoscopic findings. Over the past decade, with the introduction of newer therapeutic agents and investigation modalities such as capsule endoscopy, significant progress has been made in the diagnosis of small bowel CD . Capsule endoscopy has gained popularity not only for its use in patients with suspected CD , but also in identifying active small bowel disease in established CD and reclassifying patients with inflammatory bowel disease unclassified (IBDU ). In this chapter, we summarise the indications , diagnostic yield and subsequent management change in patients with suspected CD , established CD and IBDU undergoing capsule endoscopy. We also highlight important considerations for its use in these cohorts.
3.3.1 Background
Crohn’s disease (CD) is a transmural chronic granulomatous inflammatory disorder that can affect any segment of the gastrointestinal tract. Isolated small bowel involvement occurs in up to a third of the patients [97, 98]. Management in these individuals can be challenging due to varying symptoms, absence of raised inflammatory markers and a low sensitivity of small bowel barium study for early mucosal disease [99]. A mean delay in the diagnosis of CD of 1–7 years in published series has been reported [100, 101]. Over the past years, since the introduction of biological therapy for CD, there has been a paradigm shift in the way we manage CD with greater emphasis on mucosal healing rather than symptom control only. Mucosal healing has shown to be associated with improved long-term outcome s for CD and lower rates of hospitalisation and surgery [102]. With the advent of better diagnostic tests and imaging modalities such as capsule endoscopy , early diagnosis of small bowel Crohn’s disease (SBCD) has become ever increasingly important.
3.3.2 Clinical Features
Small bowel Crohn’s disease (SBCD) is often a difficult diagnosis to establish clinically. Patients can present with variable symptoms including abdominal pain , fatigue, diarrhoea, weight loss, with or without evidence of bleeding [103]. Patients can also present with complication s of transmural inflammation that include stricturing or fistulating disease.
Apart from GI symptoms, CD can present with extra-intestinal manifestations. These include the following:
Joint symptoms—Inflammatory arthritis mainly involving large joints
Eye symptoms—Uveitis, iritis and episcleritis
Skin manifestations—Erythema nodosum and pyoderma gangrenosum
Primary Sclerosing Cholangitis
Laboratory markers can be helpful in raising the suspicion of small bowel CD. Patients may present with iron-deficiency anaemia , B12 deficiency and/or raised inflammatory markers. It is unclear that combination of symptoms and/or laboratory markers predicts CD [104]. Faecal calprotectin (fC) has been used as a cost-effective screening tool prior to SBCE in patients with suspected CD and prior normal bidirectional endoscopy [105]. Koulaouzidis et al. [105] showed that an fC > 100 μg/g is a good predictor of positive SBCE findings, whilst fC > 200 μg/g confirmed CD in 50 % of cases. There are published guidelines on the use of SBCE in CD for USA, UK and Europe [106–108].
3.3.3 Histology
Typical histological features of CD include the presence of granulomas, areas of chronic inflammatory cell infiltrate (lymphocytes, neutrophils, plasma cell infiltrates), skip lesions, transmural inflammation with lymphoid aggregates [109]. It is, however, uncommon to see all the typical histological features of CD in a biopsy specimen [110].
Histology has always been regarded as a cornerstone in the diagnosis of Crohn’s disease . However, obtaining histological confirmation is not always possible as the disease is patchy (such that biopsies near inflamed mucosa may be normal) and may arise in less accessible areas of the intestine, such as the small bowel, leaving the clinician to decide on diagnosis and treatment without histological confirmation.
3.3.4 Investigation Modalities
3.3.4.1 Endoscopy
Ileocolonoscopy and biopsy has been the gold standard investigation for colonic Crohn’s disease. Although ileoscopy allows direct visualisation of the mucosal surface, it is limited to the distal terminal ileum (TI) in patients where TI intubation is achievable. Push enteroscopy is an alternative but is limited to the proximal small bowel and by the insertion depth of a maximum of 80–120 cm beyond the ligament of Trietz.
Endoscopic features of CD include areas of inflammation and/or ulceration adjacent to normal mucosa in a typical skip pattern along with pseudo-polyps and cobblestone mucosal appearance. These are hallmarks of the disease.
3.3.4.2 Radiology
Traditionally, small bowel follow through (SBFT) has been the investigation of choice for SBCD [89]. Although a useful tool in identifying deep ulceration, fistulae and stricturing disease, it has a low sensitivity and often misses early CD lesions [111–114], leading to a delay in initiating targeted therapy for CD [100, 115, 116].
Newer techniques such as computed-tomography enterography (CTE) and magnetic resonance enterography (MRE) have the capability of evaluating bowel wall thickness and enhancement supporting the diagnosis of CD whilst detecting extra-intestinal complication s. They have been shown in studies to have a higher diagnostic yield compared to SBFT [112, 117, 118] and are regarded as first-line radiological investigations for SB disease [107]. Both these techniques, however, rely on radiological expertise, which may not be available in all medical centres.
3.3.4.3 Capsule Endoscopy
With the introduction of capsule endoscopy in 2001, there has been a radical change in the way we image the small bowel. Two meta-analyses comparing small bowel capsule endoscopy (SBCE ) to small bowel radiology (SBR) have shown a higher diagnostic yield with results for SBCE versus SBR and SBCE versus CTE at 52 versus 16 % and 68 versus 21 %, respectively [99]. The literature on the use of capsule endoscopy can be broadly divided into the following indication s:
1.
Suspected CD : patients with no prior diagnosis of IBD undergoing capsule endoscopy
2.
Established CD : patients with CD undergoing SBCE to assess disease activity
3.
Inflammatory bowel disease unclassified (IBDU ): patients with a prior diagnosis of IBDU undergoing SBCE to reclassify their disease.
3.3.4.4 Suspected Crohn’s Disease
The introduction of SBCE as a diagnostic tool has allowed small bowel changes to be identified early in patients with previously normal radiological investigations [99, 114]. Although there is no validated scoring system for CD on SBCE, some studies have used the presence of 3 or more ulcers on SBCE to be suggestive of CD [119, 120]. Studies have suggested that by selecting patients with specific symptoms and using a strict criteria of 3 or more ulcers as diagnostic of CD, it may enhance the positive predictive value (PPV) [121, 122]. Recently, there have been studies looking at SBCE scoring systems in patients with suspected CD . There have been recent reports of higher positive predictive values, sensitivity and specificity at diagnosing CD when using the Lewis score (LS): this score, also known as the capsule endoscopy scoring index (CESI), examines 3 endoscopic parameters: oedema, ulceration and SB stenosis. Thresholds are set where a score <135 denotes a normal study and ≥790 indicates severe inflammation whilst any score between these parameters are labelled as mild activity [123]. Rosa et al. [123] showed that an LS score had a positive predictive value, negative predictive value, sensitivity and specificity of 82.6, 87.9, 82.6 and 87.9 %, respectively, for CD . Similarly, there has been another scoring system developed by Niv et al.: The Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI) [124]. Designed to measure mucosal disease activity and limit inter-observer variation, this system scores the proximal and distal segments of the study for each of the following findings: inflammation, disease extent and presence of any strictures. Each segment score is calculated by multiplying the inflammation (A) subscore with the disease extent subscore (B) and adding the stricture subscore (C). The final CECDAI score is the sum of the proximal and distal segment scores: proximal (A × B + C) + distal (A × B + C) [124]. A randomised double-blind prospective controlled study using CECDAI scoring system found a greater correlation amongst endoscopists when reporting SBCE from different study centres [125]. It must be said, however, that large clinical trials using scoring systems are lacking. In addition, Yang et al. found no statistical correlation between capsule endoscopy scoring index (CESI), clinical disease activity indices and C-reactive protein [126].
Although SBCE has high sensitivities, its specificity has not been accurately assessed [127]. The implication of false-positive tests can have major impact on patient care, not only exposing them to toxic medications, but also the psychological effects of being diagnosed with a chronic condition and a delay in diagnosing the true underlying aetiology [121].The presence of small bowel ulcers on SBCE could suggest the presence of CD ; however, studies have shown that mucosal breaks and ulcers can be present in healthy individuals in 10–15 % [125, 128–130]
There has been limited published data on the long-term follow-up of patients with suspected Crohn’s disease after SBCE . Only 3 studies have looked at clinical outcome post-SBCE [121, 122, 131]. Girelli et al. [131] were the first to publish the long-term outcome post-SBCE. The diagnostic yield was at 59 %, and although the sensitivity and specificity were 93 and 84 %, respectively, with a post-test probability of 85 % for small bowel CD, the study cohort was small with only 27 patients. Tukey et al. reported outcomes after SBCE in 102 patients with suspected CD . The study had an initial diagnostic yield of 37 % (n = 20), and within this cohort, 65 % (n = 13) had an eventual diagnosis of CD [121]. Subsequently, Kalla et al. published the largest series of 265 patients with suspected CD referred for SBCE. Their diagnostic yield was 17 % (n = 45), and of these, 69 % (n = 31) had an eventual diagnosis of CD (mean follow-up of 15 months) [122]. These studies suggest that a clinical suspicion of CD and the presence of more than 3 ulcers at SBCE can predict a clinical diagnosis in two-thirds of the patients suspected of SBCD. It is also a useful tool and does change the long-term management in patients with SBCD on capsule endoscopy ; however, strict diagnostic criteria should be adopted to minimise any false-positive results and enhance the PPV.
A negative SBCE is equally useful in patients with suspected CD . A normal result allows the clinician to exclude CD in patients with symptoms and blood parameters that may suggest otherwise thereby facilitating an early diagnosis and aiding better time and resource management amongst hospitals [122]. Although it has been shown that SBCE is a cost-effective tool for the diagnosis of small bowel CD [132], identifying the most cost-effective algorithm for SB imaging remains unclear [133].
3.3.4.5 Established Crohn’s Disease
Studies have also demonstrated that almost half of all CD patients require surgery within 10 years of diagnosis [134]. After ileal or ileocolonic resection, most patients have CD recurrence at the neo-terminal ileum [135]. It is also known that at least 20 % would require additional surgery for their recurrence [134, 136]. At present, immunosuppressive therapy is recommended in patients who are at high risk of recurrence, i.e. fistulating disease, ileocolonic disease and smokers or those with low risk of recurrence but significant endoscopic changes 6–12 months post-operatively [137, 138].
The use SBCE has been studied in patients with established CD . SBCE was found to have a diagnostic yield of 71 % for active CD compared with 35 and 39 % for small bowel radiology and CT enterography/enteroclysis, respectively [99]. Studies have also compared SBCE and MR enterography (MRE). Both modalities appear complimentary with similar diagnostic yields, yet SBCE is better at identifying mucosal disease whilst MRE is preferred for transmural and extra-intestinal changes [139–142]. There is, however, a small but definite risk of capsule retention , especially in patients with established CD . As a result, the BSG guidelines advocate the use of PillCam Patency™ prior to SBCE if abdominal pain is a significant feature [106]. Although clinical experience with PillCam Patency™ is limited, there are studies in the literature using PillCam Patency™ to detect SB stenosis. It allows an effective assessment of any luminal stenosis amongst established CD and post-operative patients [122, 143–146].
Mehdizadeh et al. [147] published the largest series on symptomatic CD patients (n = 134). Although it concluded that SBCE has a higher diagnostic yield, there was no data on long-term outcome . Recently, Long et al. (n = 86), Dussault et al. (n = 71) and Kalla et al. (n = 50) published data on the impact of SBCE on management [122, 148, 149]. All authors concluded that SBCE alters management in the majority of patients with symptomatic IBD , be it surgical resection or intensifying medical therapy.
There is limited published data on the use of SBCE to detect recurrence post-operatively [119, 122, 137]. Bourreille et al. [119] demonstrated that SBCE was inferior to ileocolonoscopy in detecting recurrence of Crohn’s disease after surgical resection (n = 32 patients), this was not supported by Pons Beltrán et al. [137]. It has been suggested that this discrepancy could be due to poor views in the distal ileum and irregular propagation of the capsule [119]. In addition, the type of anastomosis in the patient cohorts was different. Whilst one study included patients with end-to-end anastomosis, the other study had patients with side-to-side anastomosis; the latter being more difficult to examine endoscopically [119, 137]. Although these studies have allowed us to appreciate the utility of SBCE in these patients, large prospective trials are lacking.
Studies have shown that patients with established CD seem to have more extensive disease on SBCE than previously seen by ileocolonoscopy [119, 137]. Flamant et al. [150] have suggested that patients with ileal CD are more likely to have jejunal lesions and are at an increased risk of clinical relapse. However, there appears to be no correlation between symptoms and extent of small bowel disease at SBCE [151].
3.3.4.6 Inflammatory Bowel Disease Unclassified
Colonic inflammatory bowel disease (IBD) can be classified into either Crohn’s disease (CD) or ulcerative colitis (UC). There, however, appears to be a subset of patients that fall short of the endoscopic and histological criteria for CD or UC. These patients are classified as inflammatory bowel disease unclassified (IBDU ). This term was first defined by the Montreal World Congress of Gastroenterology working party in 2005 [152]. Population-based studies have shown that up to 20 % of patients with colonic IBD cannot be classified as CD or UC; this prevalence being up to 30 % amongst children [153, 154].
When colitis presentation is severe, it is pertinent to differentiate UC from CD as ileo-anal pouch anastomosis is generally contraindicated in the latter due to high risk of post-op complication s such as anastomotic leaks [155]. In addition, pouch salvage surgery is usually unsuccessful in these patients [156]. It is also postulated that the clinical course and outcome could be worse in patients with IBDU [157]. With the introduction of new biological therapies targeted at established CD or UC, confirmation of the type of IBD has become increasingly important.
There are a small number of studies, which have looked at the effect of SBCE diagnosis of CD on the management and long-term outcome in patients with IBDU [120, 122, 148, 158, 159]. Lopes et al. felt that the findings on CE did not influence management; however, their study cohort was small with a CD diagnosis of CE in 7 patients. The inclusion criterion for SBCE in their study included asymptomatic individuals with a long-standing diagnosis of IBDU . Hence, on follow-up, although SBCE diagnosed CD in 38 %, management was not altered in any of these patients as they were in clinical remission [158]. In contrast, four other studies felt SBCE was a novel and clinically useful diagnostic tool that altered management in patients with a diagnosis of CD on SBCE [120, 122, 148, 159]. All 4 studies included symptomatic IBDU patients where management was altered as a result of SBCE; this included either step up /step down medical therapy or surgery (Figs. 3.17, 3.18, 3.19, 3.20).





Fig. 3.17
Linear ulceration on capsule endoscopy

Fig. 3.18
Multiple erosions in a patient with suspected Crohn’s disease

Fig. 3.19
Luminal stenosis

Fig. 3.20
Circumferential ulceration with stenosis
Summary
Type of disease | Diagnostic yield (%) | Overall management change (%) | Important considerations |
|---|---|---|---|
17–59 (prevalence of 12–13 %) | 62 (Kalla et al.) | Diagnostic yield and prevalence dependant on strict referral and diagnostic criteria at capsule endoscopy | |
48–71 | 17–68 | Risk of capsule retention is relatively higher in this cohort, especially if symptoms of abdominal pain are present | |
17–43 | 17–73 | Diagnostic yield and post-capsule endoscopy management can vary in asymptomatic versus symptomatic individuals |
3.4 Capsule Endoscopy in Coeliac Disease
Abstract
Coeliac disease is a common autoimmune condition affecting up to 1 % of the adult population. Currently, a duodenal biopsy taken at endoscopy is required to make a conclusive diagnosis. Macroscopic changes of coeliac disease seen at endoscopy and have been shown to have high levels of specificity , especially when combined with coeliac serology. However, standard endoscopy can be poorly tolerated and is contraindicated in a small number of patients. Also changes of coeliac disease may only be apparent more distally in the small bowel than can be assessed with standard endoscopy. Complication s of coeliac disease such as ulcerative jejunitis or small bowel malignancy can also develop throughout the small bowel. This chapter will assess the role of capsule endoscopy for the diagnosis of coeliac disease in routine and equivocal cases. The role of capsule endoscopy in the investigation of non-responsive and refractory coeliac disease and small bowel malignancy will be evaluated. Capsule technology is rapidly evolving and current and potential future developments that may revolutionise capsule endoscopy in coeliac disease will be discussed.
3.4.1 Introduction
Coeliac disease is a common autoimmune condition characterised by a heightened immunological response to ingested gluten, with prevalence rates in the USA and European populations estimated to range between 0.2 and 1 % [160, 161]. Furthermore, there is some evidence to suggest that the prevalence of coeliac disease is increasing [162–164]. Finally, clinicians from both China and the Indian subcontinent are now recognising patients with coeliac disease. This had not previously been the case, and one hypothesis is that coeliac disease is emerging due to the introduction of wheat into these ethnic groups (as their diet becomes more westernised). Thus, coeliac disease is a global problem [165, 166]. The current gold standard diagnostic test for coeliac disease is small bowel histology, demonstrating the presence of villous atrophy (VA) (Marsh 3a to 3c) [167]. Corroborative evidence used to support the diagnosis of coeliac disease comes from positive serological tests (tissue transglutaminase (tTG) and endomysial (EMA) antibodies) and a clinical response to a gluten-free diet. Occasionally, when diagnostic uncertainty exists, human leucocyte antigen (HLA) typing is undertaken, which may help to exclude coeliac disease, given the high negative predictive value of this test [168].
Historically, a small bowel biopsy was obtained using a Crosby suction biopsy capsule. With the advent of fiberoptic oesophagogastroduodenoscopy (EGD), investigators were able to demonstrate that endoscopic duodenal biopsy was comparable to the suction biopsy in terms of its ability to detect VA with three or four endoscopic duodenal biopsies, taken at different levels along the duodenum and jejunum [169–171]. As well as providing a more reliable method of obtaining a small bowel sample, EGD has the advantage over Crosby capsule in that it allows direct visualisation of the duodenal mucosa. Investigators are now able to detect the endoscopic markers of VA—reduction or absence of Kerckring’s folds, mosaic mucosal pattern, micronodular pattern, scalloping and possibly duodenal erosions [172–175]. Although these markers can be seen in other conditions that cause VA, they have excellent specificity when combined with positive coeliac serology.
However, there are several potential limitations of EGD as part of this diagnostic pathway. These include its invasive nature and its inability to evaluate small bowel mucosa beyond the duodenum. Changes of coeliac disease are well recognised to be patchy [176], and occasionally, in some patients, the small bowel distal to the reach of a standard gastroscope may be more affected than the proximal bowel where biopsies are taken [177–179]. In addition, duodenal biopsy sampling may be affected by specimen orientation [180]. The distribution of complication s of coeliac disease such as ulcerative jejunitis and enteropathy-associated T-cell lymphomas is also particularly important as these appear to be more commonly seen in the distal small bowel [181–183]. For this reason, other endoscopic modalities such as push or double-balloon enteroscopy (DBE) may be employed in order to allow more extensive evaluation of the small bowel and obtain histology [177, 184]. However, these investigations are offered in relatively few centres that are labour intensive and are more invasive than standard EGD.
Capsule endoscopy (CE) could therefore provide a useful alternative for direct visualisation of the small bowel. CE compares favourably with other small bowel imaging techniques such as magnetic resonance imaging (MRI) enteroclysis (Van Weyenberg et al. 2013). CE is a well-tolerated, minimally invasive test, predominantly utilised for the assessment of obscure gastrointestinal bleeding , inflammatory bowel disease and polyposis syndromes . However, there has been increasing interest in the role CE may have in coeliac disease. With an eightfold magnification power comparable to a dissecting microscope, capsule endoscopy has the potential to detect VA and other small bowel complication s seen in coeliac disease. In this chapter, we will discuss the potential role of capsule endoscopy in the diagnosis of coeliac disease, its use in complicated cases and the potential for future development .
3.4.2 Endoscopic Markers of Coeliac Disease
A number of endoscopic markers for coeliac disease have been identified. The presence of these markers at EGD is sometimes used to determine whether duodenal biopsies are indicated. However, at present, capsule endoscopes are unable to take biopsy samples, so what are the endoscopic markers of coeliac disease? How accurate are they in diagnosis? and Are they applicable to CE? Endoscopic markers of coeliac disease include the following: reduction or absence of Kerckring’s duodenal folds; scalloping, which is a notched and nodular appearance of the duodenal folds; increased visualisation of submucosal vasculature; a mosaic pattern, resulting from the cobblestone or micronodular appearance of the mucosal surface; and mucosal fissures and grooves [173]. Endoscopic images of these features are shown in Fig. 3.21. Other endoscopic features such as duodenal erosions have been suggested but are not widely reported and can be seen in multiple other conditions [174]. A wide range of studies have attempted to demonstrate the usefulness of endoscopic markers during EGD with contradictory results. Compared to the gold standard of histology the sensitivity and specificity of all endoscopic markers combined varies from 37 to 94 % and from 92 to 100 %, respectively [185–188]. There are several possible explanations for the absence of endoscopic markers in patients with coeliac disease. Although histology is the gold standard test, it is known that this does not have 100 % sensitivity particularly if small numbers of biopsies are taken or enteropathy is patchy and if specimens are poorly orientated [176, 180]. Also endoscopic markers might actually be absent for degrees of enteropathy milder than subtotal or total VA [185]. Although coeliac disease is the most common cause of VA, particularly in patients with positive coeliac serology, the specificity of endoscopic markers of coeliac disease is also not 100 % and there are a number of differential diagnoses VA [189]. These include infections such as giardiasis and Whipple’s disease or other autoimmune conditions such as Crohn’s disease or autoimmune enteropathy. A full list of differential diagnoses is shown in Table 3.3 with capsule images of some of these differentials shown in Fig. 3.22. Particular consideration should be made to these differentials in CE as there is no confirmatory intestinal biopsy. The lack of a biopsy is also important when we are considering the wide range of reported sensitivities of endoscopic markers for coeliac disease. The potentially low sensitivity of these markers may lead to a number of missed diagnoses. What is the evidence, therefore, that CE is a suitable tool for diagnosing or assessing coeliac disease? The potential indication s for CE in coeliac disease are shown in Table 3.4 and will be discussed in detail below.



Fig. 3.21
Endoscopic images showing signs of villous atrophy. 1 Scalloping. 2 Cobblestone appearance. 3 Mosaic pattern and scalloping. 4 Increased visible vessels and loss of duodenal folds
Table 3.3
Differential diagnoses for villous atrophy
Agammaglobulinaemia |
Amyloidosis |
CMV enteritis |
Coeliac disease |
Collagenous sprue |
Chronic gastroenteritis |
Crohn’s disease |
Cryptosporidium infection |
Eosinophilic enteritis |
Giardiasis |
Graft-versus-host disease |
HIV enteropathy |
Intestinal lymphangiectasia |
Ischaemia |
Mastocytosis |
Radiation enteritis |
Small bowel bacterial overgrowth |
Tropical sprue |
Tuberculosis |
Whipple’s disease |
Zollinger–Ellison syndrome |

Fig. 3.22
Capsule images of potential differential diagnoses for villous atrophy. 1 Giardiasis—notching of oedematous duodenal folds. 2 Autoimmune enteropathy—flat mucosa but no other features of coeliac disease. 3 Crohn’s disease —mosaic patternation and scalloping seen distally but not proximally. 4 Chronic norovirus infection in an immunosuppressed patient confirmed by PCR in serum and histology—note markedly oedematous folds
Table 3.4
Indications for capsule endoscopy in coeliac disease
Suspected coeliac disease in patients unwilling to undergo EGD |
Assessing extent of disease and response to treatment |
Equivocal cases of possible coeliac disease |
Investigation of non-responsive coeliac disease |
Exclusion of enteropathy-associated lymphoma and ulcerative jejunitis in patients with refractory coeliac disease |
3.4.3 Suspected Coeliac Disease
Diagnostic EGD with duodenal biopsies has an excellent safety record and is the gold standard test for diagnosing coeliac disease. However, even with adequate sedation, EGD can be poorly tolerated with a small risk of serious complication s such as perforation. As a result, some patients are unwilling or unable to undergo the procedure. CE is much better tolerated by patients and as a result may be a potential imaging modality in these patients. CE may also have some other potential advantages over EGD in assessing macroscopic features of coeliac disease. CE is performed without the air insufflation, required for standard endoscopy, which may obscure subtle changes of VA. The optical dome of the capsule is in close contact with the mucosa, which may allow better visualisation of the mucosa. This has a similar effect to water immersion endoscopy, which has been shown to have excellent sensitivity and specificity during standard endoscopy of unselected patients [190]. Characteristic changes of coeliac disease in some patients may only be seen distally, and CE also allows visualisation of the entire length of the small bowel. Capsule images of villous atrophy are shown in Fig. 3.23.


Fig. 3.23
Capsule images showing a mosaic pattern and b scalloping
A small study of 10 coeliac patients with VA and 10 controls was the first to assess the utility of CE in diagnosing coeliac disease [191]. All of the images were reviewed by 4 blinded investigators 2 of who had extensive pre-study experience of reporting CE and demonstrated 100 % accuracy in identifying patients with VA. The 100 % accuracy is particularly impressive given that 4 out of the 10 patients with VA had apparently normal looking duodenal mucosa at EGD. However, there was relatively poor inter-observer agreement between some of the investigators with kappa coefficients (κ) as low as 0.26. When results were combined with those of the less experienced clinicians, they achieved a sensitivity , specificity , positive predictive value (PPV) and negative predictive value (NPV) of 70, 100, 100 and 77 %, respectively. There are several limitations to this study with a small sample size, high degree of ascertainment bias and only one patient included who had partial VA (Marsh 3a). Some of these limitations were addressed in a study of 21 EMA-positive patients and 23 antibody-negative controls [192]. The EMA-positive patients had a range of histology findings from Marsh 1 to 3c including 1 patient with lymphocytic duodenosis (Marsh 1) only and 5 patients with partial VA (Marsh 3a). All of the control patients had normal histology. Seventeen of the 20 patients with VA were correctly identified via CE, and there were no false positives in the control group. A further study of 22 patients with positive serology 8 of whom had normal duodenal histology showed that results were similar when tTG was used for patient selection with sensitivity, specificity, PPV and NPV for CE of 93, 100, 100 and 89 %, respectively [193]. Rondonotti et al. [194] also identified patients with positive serology and showed a sensitivity of 89 % for in 28 patients with confirmed villous atrophy. Finally, in the largest study to date of 37 patients with coeliac disease and 38 gender-matched controls, CE showed a sensitivity of 92 % and specificity of 100 % [178]. Importantly, the disease group included 19 patients with partial VA.
A consistent finding in all of these studies is that the PPV and specificity in the presence of EMA or significantly elevated tTG (usually greater than ten times the upper limit of normal) for the recognition of endoscopic markers of coeliac disease is 100 %. However, the high pretest probability of coeliac disease in all of these studies may again be a potential limitation, leading to an overestimation of CE performance. However, they accurately reflect real-life clinical practice where patients are likely to be selected for CE of the basis of positive serology and suggest that CE may be an appropriate tool for patients who are unable to undergo EGD. A summary of the studies in suspected coeliac disease is shown in Table 3.5.
Table 3.5
Summary of studies of utility of capsule endoscopy in diagnosing coeliac disease
Year | Country | Patients | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
2005 | Canada | 10 coeliac disease patients and 10 controls | 70 | 10 | 100 | 77 |
2007 | USA | 32 coeliac disease patients 11 controls | 87.5 | 90.9 | 96.5 | 71.4 |
2007 | UK | 21 EMA-positive patients 20 controls | 85 | 100 | 100 | 88.9 |
2006 | Italy | 10 untreated coeliac disease, 10 RCD, 3 treated coeliac disease, 2 EATL, 1 potential coeliac disease, 6 controls | 90.5–95.2 | 63.6 | 100 | 77.8–87.5 |
2008 | USA | 38 untreated coeliac disease patients, 38 controls | 92 | 100 | 100 | 93 |
2008 | Turkey | 8 untreated coeliac disease patients | 100 | 100 | NA | NA |
2011 | Australia | 14 coeliac disease patients and 8 EMA-positive patients with normal duodenal biopsy | 86 | 100 | 100 | 80 |
3.4.4 Assessing Extent of Disease and Response to Treatment
One area where CE may confer an advantage over standard endoscopy is that CE has the potential to image the entire small bowel. The gluten load is highest in the proximal small bowel but the entire length of the small bowel can be affected as demonstrated in a study of terminal ileal biopsies [195]. Patients with newly diagnosed coeliac disease had significantly higher intraepithelial lymphocyte counts when compared to controls. However, not all coeliac disease patients were affected. It would seem intuitive that the more of the bowel that is affected, the more severe symptoms and the higher the chance of potential complication s. However, this has not been proven mainly because it is difficult to assess the extent of disease. Capsule endoscopy may provide a way of doing this.
Also if CE for newly diagnosed coeliac disease patients was common practice, then there is potential for both assessing disease severity response to treatment. This may be pertinent in patients with persistent symptoms as it is known that the villous architecture recovers more quickly in the distal small bowel and proximal duodenal biopsies may show continuing VA. These patients may be labelled as non-adherent. However, the lack of improvement in histology proximally may not represent a significant improvement in the extent of disease. In a recent study of 38 untreated coeliac disease patients and 38 controls, the authors attempted to assess extent of disease by 2 methods [178]. Firstly, the investigator reviewing the images was invited to make a qualitative assessment of severity based on whether disease was patchy or continuous and extent of disease. A second quantitative assessment of severity was also used. The total length of time with changes of VA and time relative to the small bowel transit time were recorded. The authors were unable to show a relationship between either qualitative or quantitative measurements of extent of disease and severity of clinical presentation; however, a positive EMA was associated with more extensive disease. In the 30 coeliac disease patients who agreed to repeat CE after GFD, the mean time with abnormality reduced from 60 to 12 min. A second more recent study of 12 patients with coeliac disease who had repeat CE after 12 months on a GFD has also demonstrated this improvement. The investigators assessed the extent of disease as a percentage of the total small bowel transit time [193]. Although there was no initial correlation between extent of disease and clinical severity, they did demonstrate a significant reduction in the mean time with VA.
These 2 studies have so far failed to demonstrate any relationship between extent of small bowel involvement and clinical severity of disease. However, as experience with CE in coeliac disease increases, this may become possible. The use of CE to assess small bowel healing does appear to be a promising area; however, this will only become relevant as more patients undergo CE at the time of diagnosis. New technologies as discussed later in this chapter may also help to improve the objective measurement of disease severity.
3.4.5 Equivocal Cases
Another area where CE may play a role is in the investigation of equivocal cases of coeliac disease. As previously discussed, the changes of coeliac disease can be patchy and a duodenal biopsy in patients with positive serology may not demonstrate VA. Lesser degrees of histology that can be associated with coeliac disease are non-specific and are seen in a variety of other conditions. This can leave some patients without a definitive diagnosis. How therefore should we investigate patients with positive coeliac serology but normal or non-specific duodenal histology? An international consensus conference on the use of capsule and double-balloon enteroscopy advocated the potential use of CE in this situation [196]. There is, however, limited data assessing the role of CE in these equivocal cases.
In a large multicentre study, the investigators identified patients with gastrointestinal symptoms and positive EMA, tTG or antigliadin antibodies (AGA) [194]. Duodenal biopsies and CE were performed at the time of presentation, and 11 patients were found to have normal duodenal histology. Of these, 10 patients also had a normal capsule endoscopy ; however, these patients were all positive for AGA only which is non-specific and seen in a variety of other conditions. The one patient that had evidence of VA on capsule endoscopy was also positive for EMA and had biopsy-proven dermatitis herpetiformis making coeliac disease very likely. One patient with Marsh 1 changes and positive EMA had a normal CE; however, the 3 patients with Marsh 2 changes and positive EMA and tTG all had evidence of VA on CE. The authors do not state whether any further confirmatory tests such as HLA genotyping or further small bowel biopsies were taken to confirm coeliac disease but it is likely that these were additional diagnoses that had been missed by conventional EGD and duodenal biopsy.
There is, however, conflicting evidence. In a study of 8 patients with positive serology (EMA or tTG) and a normal duodenal biopsy, CE did not reveal any endoscopic features of coeliac disease [193]. Thus, the investigators concluded that there was no benefit in performing CE for this subgroup of patients. In a further study, 22 irritable bowel syndrome patients with positive AGA, EMA or tTG and normal duodenal histology underwent CE and HLA genotyping [197]. Subtle mucosal abnormalities within in the small bowel, such as mucosal breaks, ulceration or denuded and blunted villi, were seen in 55 % (12/22) of cases. However, the authors felt that none of these features were conclusively characteristic of coeliac disease and these changes were seen in both patients with and without an HLA genotype required for coeliac disease. The majority of the patients only had a positive AGA, which as discussed previously has a low specificity for coeliac disease. The single patient with a positive EMA (the most specific marker for coeliac disease) had a normal CE. Finally, in a study of 30 patients with Marsh 1 or 2 changes, only 6 of whom had positive EMA or tTG, 1 patient was diagnosed with coeliac disease and another with small bowel Crohn’s on the basis of CE appearances [198]. It is clear that further work is required to assess the cost-effectiveness of the use of CE in these equivocal cases if the yield is as low as in this final study. CE use may be justified however, in EMA or tTG positive patients with Marsh 1 or 2 changes or gastrointestinal symptoms particularly, if they are unwilling to undergo further EGD and repeat biopsies.
Another diagnostic challenge is antibody-negative VA. As previously discussed, there is a wide range of differential diagnoses for VA. In the study of equivocal cases by Kurien et al. [198], they also included a group of patients with antibody-negative VA to see whether this increased the diagnostic yield. Patients were extensively investigated for coeliac disease including HLA phenotyping, by monitoring response to GFD and, in some cases, repeat duodenal biopsies. On the basis of CE appearances and other ancillary tests, 7 patients could be diagnosed with coeliac disease and 2 further patients were diagnosed with small bowel Crohn’s as a cause for VA. Again, this is a single small study and further work needs to be done to clarify the role of CE in antibody-negative VA cases. This is particularly important as CE alone is probably insufficient to confirm a diagnosis of coeliac disease as endoscopic markers are not specific to coeliac disease rather they are predictors of mucosal disease [199].
3.4.6 Non-responsive Coeliac Disease, Refractory Coeliac Disease and Enteropathy-Associated T-Cell Lymphoma
Although the majority of patients with diagnosed coeliac disease improve on a gluten-free diet (GFD), up to 30 % patients do not respond as expected. In many of these patients, other causes for their symptoms such as microscopic colitis, small bowel bacterial overgrowth or pancreatic insufficiency are identified [200]. However, many of these patients undergo repeated endoscopy and duodenal biopsy to assess small bowel healing and look for serious complication s such as refractory coeliac disease (RCD) or enteropathy-associated T-cell lymphoma (EATL). RCD is defined as persistent malabsorptive symptoms and villous atrophy despite strict adherence to a GFD. It is subdivided into types I and II depending on clonality of intraepithelial lymphocytes. RCD type II carries a worse prognosis and is associated with greater progression to EATL [200]. However, changes may not be confined to the proximal small bowel and may be out of the reach of a standard gastroscope. CE may therefore play a role in the investigation of these patients.
Several studies have attempted to delineate a role for CE in non-responsive patients. In blinded comparison of duodenal biopsy and CE in 18 patients who had failed to respond to a GFD, 67 % of those with histological evidence of coeliac disease had abnormal CE [201]. Six patients with normal histology also had a normal CE. Four patients, however, had evidence of persistent histological changes of coeliac disease but had normal CE appearances. Agreement between histology and CE appearances was therefore fairly modest with a κ coefficient of 0.65. Importantly, however, 2 cases of ulcerative jejunitis were identified. Ulcerative jejunitis is usually associated with RCD type II that can result in small bowel stricturing and is associated with a high risk of developing EATL. Early identification of RCD type II may allow effective treatment with immunosuppression and prevent progression to EATL. These findings were replicated in a recent study of 69 patients with coeliac disease and persisting symptoms [198]. Signs of VA were identified in 45 %, and serious complication s were identified in 8 patients including 2 cases of EATL, 1 case of ulcerative jejunitis and 4 RCD type 1. However, although the investigators were blinded to the EGD findings, no control groups were included in either of these studies, leading to a high degree of ascertainment bias, which may have overestimated the usefulness of CE. This was addressed in a recent study of 42 non-responsive patients, 84 controls and 30 patients with uncomplicated coeliac disease who had responded well to a GFD for at least 6 months [182]. Only 9 of 16 patients with villous atrophy on histology had abnormal findings on CE. Four patients with normal histology had apparently abnormal CE appearances. As a result, overall the agreement between histology and CE findings was weak with a κ coefficient of 0.44. However, it must be noted that duodenal histology may be a less than perfect gold standard as changes can be patchy. It may be the findings on CE represented VA that was distal to the reach of EGD and standard duodenal biopsy. Also it must be noted that of the 16 patients with VA, 13 had partial VA (Marsh 3a), which may not be seen as clearly as total VA. All 3 of the most severe lesions were correctly identified as abnormal by CE. Again, a case of RCD type II was identified by CE and confirmed by DBE and biopsy. The use of CE to assess the extent and severity of disease in patients with known RCD may also be helpful as shown in a recent study of 29 patients with RCD and 9 patients with symptomatic coeliac disease [202]. Three cases of EATL and 5 cases of ulcerative jejunitis requiring specific treatment in the RCD cohort were identified. The majority of the RCD patients also underwent DBE, and the authors concluded that 17 patients could have avoided this invasive investigation based on CE findings.
Apart from this final study, where there was an unusually high proportion of patients with RCD, the apparent diagnostic yield for complication s such as EATL and ulcerative jejunitis appears low. However, these diagnoses carry significant rates of morbidity and mortality, which may be reduced by prompt diagnosis. The use of capsule in non-responsive patients may therefore be justified. Patients with ulcerative jejunitis and EATL can have a significant risk of small bowel stricturing. CE should therefore be used with caution, and a patency capsule should always be employed to reduce the incidence of capsule retention . Capsule images of ulcerative jejunitis and EATL are shown in Figs. 3.24 and 3.25.



Fig. 3.24
Ulcerative jejunitis. Capsule appearance from a patient with known coeliac disease and persistent symptoms on a gluten-free diet. 1 shows normal proximal small bowel. 2 shows a complete loss of villi and ulceration consistent with ulcerative jejunitis

Fig. 3.25
Enteropathy-associated T-cell Lymphoma
3.4.7 New Technology and Future Development
As previously discussed, the main drawback of CE is the inability to perform biopsy. It may, however, be possible to incorporate this function into future incarnations of capsule technology [203]. In the near future , these advances, however, are only likely to allow non-targeted biopsies of the small bowel. As previously discussed, changes of coeliac disease can be patchy and biopsies may miss affected areas. Another method therefore of increasing the diagnostic yield would be to incorporate other image-enhancing techniques. Multiple techniques such as narrow-band imaging or optical-band imaging, amongst others, have been used in conventional endoscopy with limited improvement in diagnostic accuracy [204]. Capsule has already been shown to be more accurate than standard endoscopy for identifying macroscopic features of VA, and as capsule technology and picture resolution improve, these techniques may be incorporated and may prove beneficial in increasing the yield further.
These technologies are, however, not presently available and may never come to fruition. An interesting development that is presently undergoing clinical trials is the use of digital quantitative analysis of CE images. CE interpretation is currently subjective as has been demonstrated by the significant inter-observer variability in some studies. CE can miss lesions as demonstrated by a pooled analysis of 530 examinations where 10.8 % of lesions picked up on conventional endoscopy were missed by CE [64]. There is also a lack of standardisation of severity of CE findings. An objective computerised analysis of CE images would therefore seem advantageous. This has been developed using current CE technology and involves the production of a 3-dimensional representation of 2-dimensional CE images, mucosal protrusions can then be assessed for height, width and number per image. The images are also converted to greyscale and the intensity of each pixel is measured. These techniques were used in 3 recent studies [205–207]. On analysis of these images, patients with villous atrophy showed blunted protrusions with lower height and greater diameter. The mucosal surface also showed greater variability in pixel greyscale intensity in patients with VA, which the authors postulated was due to fissures, scalloping and mosaic pattern seen in patients with VA. Digital enhancement techniques have also been used to estimate small bowel motility, which is known to be impaired in patients with active coeliac disease [208]. With further development, these techniques may prove invaluable in the assessment of patients with suspected or known coeliac disease. Crucially, this objective measurement may also allow for more accurate quantification of severity and improvement in pathology on a GFD.
3.4.8 Conclusions
At present, duodenal biopsy remains the gold standard for diagnosing coeliac disease; however, CE may play a role in those patients who are unable or unwilling to undergo standard EGD and biopsy. CE is also proving an important diagnostic tool for investigating patients for possible complication s of coeliac disease such as small bowel malignancy, RCD and ulcerative jejunitis. There are potential limitations to CE with high degrees of inter-observer variability, potentially high miss rates for significant lesions and the risk of capsule retention in patients with strictures resulting from complicated coeliac disease. However, technology is evolving and future development s may allow small bowel biopsy or incorporate ‘virtual biopsy’ technologies that will improve the versatility of CE in coeliac patients.
3.5 Polyposis Syndromes
Abstract
Hereditary gastrointestinal (GI) polyposis syndromes are rare autosomal dominant disorders that are characterised by the presence of GI polyps, extra-GI phenotypic manifestations and an increased risk of GI and extra-GI neoplasia. The histopathology of polyps, nature of other phenotypic manifestations and degree of risk of malignancy are governed by the genetic mutations of the underlying syndrome. Small bowel (SB) capsule endoscopy (SBCE ) has a role to play in the surveillance and management of patients with SB polyposis, and this role is best established for patients with Peutz–Jeghers syndrome (PJS ). Although polyps in PJS have distinct hamartomatous features, large (≥1.5 cm) lesions contribute to a major part of the disease burden in patients with PJS by their potential to cause SB intussusception and GI bleeding. Surveillance strategies for the detection of clinically significant PJS SB polyps by minimally invasive investigations such as SBCE and magnetic resonance enterography (MRE) are therefore employed to reduce the risk of these complications by facilitating pre-emptive removal of polyps by device-assisted enteroscopy (DAE) or elective surgery. The role of SBCE in the surveillance of patients with other polyposis syndromes such as familial adenomatous polyposis (FAP) is less clearly defined.
Keywords
Small bowel polyposis · Polyps · Capsule endoscopy · Peutz–Jeghers syndrome · PJS · Familial adenomatous polyposis · FAP · Enteroscopy
3.5.1 Introduction
Hereditary gastrointestinal (GI) polyposis syndromes are rare autosomal dominant disorders that are characterised by the presence of GI polyps, extra-GI phenotypic manifestations and an increased risk of GI and extra-GI neoplasia. The histopathology of polyps, nature of other phenotypic manifestations and degree of risk of malignancy are governed by the genetic mutations of the underlying syndrome. Small bowel (SB) capsule endoscopy (SBCE ) has a role to play in the surveillance and management of patients with SB polyposis. Although this is now established for patients with Peutz–Jeghers syndrome (PJS), evidence to support the routine use of SBCE for the surveillance of patients with other polyposis syndromes is lacking.
3.5.2 Peutz–Jeghers Syndrome
Peutz–Jeghers syndrome (PJS ) is a high-penetrance autosomal dominant polyposis syndrome, which is associated with a germline mutation in the STK11/LKB1 gene (19p13.3) in up to 94 % of cases [209]. PJS has an incidence of about 1 in 8,500 to 1 in 200,000 live births [210–213] and is characterised by a phenotype that includes the presence of distinct mucocutaneous melanin pigmentation, gastrointestinal (GI) polyposis [214] and a predisposition to GI and extra-GI malignant disease [210, 212–218].
PJS polyps are thought to arise as a result of GI mucosal prolapse [211, 216] and have a characteristic hamartomatous histopathology including ‘arborisation’ of a smooth muscle core with ‘frond-like’ epithelial lengthening (Fig. 3.26). Although PJS polyps may occur anywhere within the GI tract, they occur predominantly within the proximal small bowel (SB) [214, 218, 219]. PJS SB polyps that are ≥1.5 cm in size are considered to be clinically significant and contribute to a major part of the disease burden in patients with this condition, mainly by resulting in intussusception and SB obstruction , often requiring emergency surgery [211–213, 218, 220, 221]. SB complication s arising from large PJS polyps frequently result in multiple laparotomies throughout a patient’s lifetime [222]. These complication s commence in childhood and early youth [211, 223–225], and the cumulative risk of SB intussusception may be as high as 50 % by the age of 20 years [218].


Fig. 3.26
Scanning view of a PJS polyp(ileum). Haematoxylin and eosin (H&E) stain a shows a large, pedunculated, lobulated polyp, supported by broad bands of muscularis mucosae smooth muscle. Smooth muscle actin (SMA) immuno-stain b highlights the tree-like branches of smooth muscle extending from the muscularis mucosae into the periphery of the polyp; the fibrous bands are thicker centrally (images courtesy of Dr TuVinh Luong, Academic Department of Cellular Pathology, The Royal Free Hospital and UCL School of Medicine, London)
In view of the high risks associated with clinically significant SB polyps (≥1.5 cm), current guidelines recommend that patients with PJS aged ≥8–10 years, should undergo SB surveillance using a minimally invasive modality such as SB capsule endoscopy (SBCE ) and/or radiological diagnostic imaging [magnetic resonance enterography/enteroclysis (MRE)] on a biennial to triennial basis [211, 220, 221] in order to facilitate early detection of large PJS polyps and also to investigate for possible occult SB malignancies. SBCE is very well tolerated by patients and is a less invasive option for the surveillance of the SB in patients with PJS [220]. The limitations relating to SBCE include its potential to miss clinically significant polyps, especially if these are located within the proximal SB [220, 221, 226–228] and challenges relating to the estimation of polyp size and location and (Fig. 3.27), possible ‘double counting’ of polyps [220, 221]. Some of these limitations (such as potential miss rates) may be mitigated by the more advanced technology of newer generation capsules, such as the PillCam SB3® (Given Imaging , Yoqneam, Israel), which also incorporates an ‘adaptive frame rate’, enabling a higher frequency of frame capture (up to 6 frames per second) during rapid capsule transit.


Fig. 3.27
A variety of PJS SB polyps as seen at video capsule endoscopy (VCE) (PillCam™ SB2, Given Imaging , Yokneam, Israel). Polyp tissue is usually darker than the surrounding SB mucosa (blue arrows); estimation of polyp size and number can be challenging and often only a small portion of larger polyps can be identified (images courtesy of Ms Aine O’Rourke and Dr Chris Fraser, St Mark’s Hospital and Academic Institute, London)
Before the introduction of SBCE and MRE into clinical practice, SB surveillance in patients with PJS depended on SB follow through (SBFT). However, SBFT is limited by low sensitivity , poor spatial resolution (due to its two-dimensional quality), with poor differentiation of overlapping SB loops and also exposes patients to a significant dose of ionising radiation [220, 229–231]. Radiological imaging of the SB in patients with PJS has therefore been superseded by MRE [220, 221]. A recent prospective comparative study of MRE versus SBCE for this indication showed that although patients tolerated SBCE better and preferred it to MRE [220], the 2 modalities were comparable for the detection of clinically significant (≥1.5 cm) PJS SB polyps. Currently, MRE may also allow for more accurate estimation of PJS polyp size and respective polyp location (Fig. 3.28) [220, 221, 232] and therefore appears to be a useful, alternative modality to SBCE for surveillance or for further evaluation of polyps detected by SBCE [220, 221].


Fig. 3.28
A large (2.5 cm) PJS ileal polyp (red arrow) as seen at magnetic resonance enterography (MRE) (image courtesy of Dr Arun Gupta, St Mark’s Hospital, London)
Minimally invasive SB surveillance for clinically significant (≥1.5 cm) polyps allows pre-emptive removal, before an episode of intussusception occurs, potentially avoiding the need for emergency surgery and possible SB resection [211, 222]. This minimally invasive approach to the management of PJS SB polyposis has been enhanced by the introduction of device-assisted enteroscopy (DAE). DAE facilitates endoscopic excision of PJS polyps located deep within the SB (Fig. 3.29) [233–243] and therefore has the potential to obviate the need for laparotomy with intra-operative enteroscopy (IOE), which until recently was the sole option available for removal of PJS polyps deep within the SB [244, 245]. Laparotomy with IOE, however, exposes patients to major abdominal surgery and its potential complication s such as post-operative intra-abdominal adhesions and short-bowel syndrome [246–248]. Data on DAE for this indication mainly relate to the double-balloon enteroscopy (DBE)and suggest that this endotherapeutic modality may offer a minimally invasive alternative to laparotomy and IOE for selected patients with PJS [216, 219, 236–238, 240]. The introduction of a DAE-based therapeutic strategy in childhood (before the patient’s first laparotomy has been required) may enhance its success, since DAE may be hindered by the presence of post-surgical intra-abdominal adhesions [249, 250]. In patients who have already developed post-surgical adhesive disease, division of adhesions by mini-port laparoscopic assistance during DAE may still provide a less invasive alternative to laparotomy with IOE for SB polypectomy [251]. However, in certain patients, laparotomy with IOE shall continue to have a major role to play in the management of SB polyps and the overall strategy should be tailored to the requirements of the individual patient. The current recommendations for the surveillance and management of PJS SB polyposis using minimally invasive strategies are presented as an algorithm (Fig. 3.30).










