Small and Large Bowel Structure: Developmental and Mechanical Disorders
STRUCTURE OF THE SMALL AND LARGE INTESTINES
External Appearance
Small intestine. The adult small intestine ranges from 300 to 900 cm in length, depending upon the tone and the degree of stretch induced when measurements are taken. The duodenum constitutes the most proximal portion, is approximately 25 cm in length, and, except for its distal fourth portion, is retroperitoneal and fixed. The duodenum extends from the first portion (duodenal bulb) downward to the second portion on the right side of the spine, to the level of the fourth lumbar vertebrae. The second portion of the duodenum is often referred to as the descending duodenum. The third portion traverses across the spine to complete a C shape that embraces the head and body of the pancreas. The common bile duct and the main pancreatic duct (of Wirsung) usually empty into a common channel, opening at the ampulla of Vater on the medial wall of the descending duodenum. An accessory pancreatic duct (of Santorini) opening is present in up to 70% of patients. It is located 2 to 3 cm proximal to the ampulla of Vater and is the exit for the accessory (minor) duct. The region of the duodenojejunal junction is a fixed landmark fluoroscopically and is referred to as the ligament of Treitz. At this junction, the duodenum becomes unfixed and wrapped in a mesentery.
The proximal 40% of the mobile small intestine is arbitrarily designated as the jejunum and the distal 60% as the ileum. In barium radiographs, the jejunum is concentrated in the left upper quadrant, the jejunoileal region in the midabdomen, and the terminal segments of the ileum in the right lower quadrant. There are some gross differences between the jejunum and ileum. The proximal jejunum is up to 3.5 cm in diameter, or one and one-half to two times as large as the ileum, which measures up to 2.5 cm in diameter. The mesentery of the ileum contains more adipose tissue compared to jejunum. Ileum lies mostly in the lower abdomen and pelvis while jejunum lies in the upper abdomen.
Large intestine. The large intestine comprises that segment of the bowel distal to the ileocecal valve (ICV). It is “large” because of its diameter relative to that of the “small” intestine, the circumference being approximately double. On a daily basis, about 1 L of fluid enters the colon from the ICV, but this is reduced to about 100 mL, which is eliminated. The colon is therefore an absorptive organ for water, electrolytes, bile salt, and other substances produced by bacterial degradation; it also has complex mixing mechanisms as well as acting as a convenient storage facility. It must also resist huge numbers of organisms of different types, some invasive and some toxin producing, as well as a variety of chemicals and potential carcinogens. It begins at the ICV and consists of the right, transverse, and left colon, the transitions between them being at the hepatic and splenic flexures, respectively. The right colon consists of the cecum, which is the saccular part of the large bowel below a horizontal line at the ICV from which the appendix arises, and the ascending colon. The left colon consists of the descending and sigmoid colon together with the rectum.
The large bowel is about 120 to 150 cm in length and characterized externally by its large diameter, the presence of three bands of longitudinal muscle, 0.6 to 1.0 cm in width, running through most of its length, the tenia coli, and the fatty appendices epiploicae. As it passes across the pelvic brim, the descending colon becomes the sigmoid colon, the latter ultimately becoming the rectum at the sacral promontory.
The orifice of the appendix is usually found immediately (1-3 cm) below that of the ICV; the gross and microscopic appearances of the appendix are discussed in greater detail in Chapter 17.
The ascending colon is about 15 to 20 cm in length, running up to the hepatic flexure, at which point it becomes the transverse colon. The latter is 30 to 60 cm in length and is somewhat U shaped, the definition of the U being governed by its length and that of its mesentery, by which it is suspended and dips toward the pelvis. At the splenic flexure, it becomes retroperitoneal and fixed, and passes down toward the pelvis as the descending colon, which is about 20 to 25 cm in length. The sigmoid colon is the natural continuation of the descending colon, beginning approximately where the large bowel crosses the pelvic brim. However, it is also characterized by the presence of a mesentery, although its length, and consequently the length of the sigmoid colon, is very variable, measuring between 20 and 85 cm, with an average of about 40 cm. At the third sacral vertebra, and corresponding roughly to the level of the levator ani muscles at the superior aspect of the internal anal sphincter, the mesentery of the sigmoid colon terminates and the sigmoid colon becomes the rectum. It continues for 10 to 15 cm to the dentate line, which traditionally marks the anatomic anorectal junction. The rectum is longer in men than in women.
The external surface of the large bowel is characterized by the presence of three longitudinally running teniae coli, which are localized condensations of the longitudinal muscle coat and are about 0.5 cm in width. It should be noted that the entire large bowel has a complete external longitudinal muscle coat throughout its length, the teniae being thickenings within this muscle layer. One is close to the attachment of the mesentery, sometimes known as the mesocolic tenia; the other two are antimesenteric, and are almost equidistant from each other and from the mesenteric tenia. One is known as the free tenia (tenia libera), and is easily seen on the anterior surfaces of the ascending and descending colons (Fig. 16-1). The other is the epiploic tenia. It is best seen on the anterior surface of the transverse colon, which folds over anteriorly at the flexures, exposing this tenia. Proximally, the three teniae unite at the base of the appendix, which they completely invest. Distally, at the rectum, all three teniae flare out, forming an external muscle coat similar to that of the small intestine. Blood vessels penetrate the bowel wall on each side of the tenia.
The external surface of the large intestine is also characterized by a series of appendices epiploicae, which are pockets of fat hanging from the bowel, their size correlating with the degree of fat found elsewhere in the body (Fig. 16-1). In the ascending and descending colon, two rows are usually present; in the transverse colon, a single row is found adjacent to the tenia libera on its undersurface. The transverse colon also has the greater omentum hanging from it and the lesser omentum passing up superiorly toward the stomach.
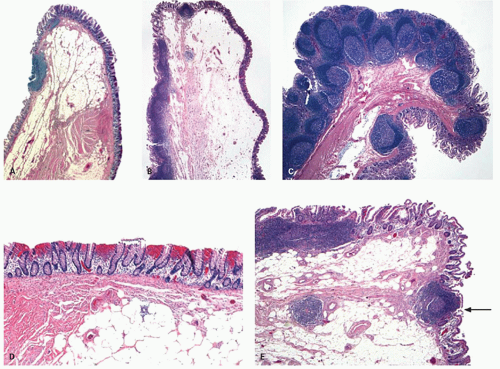
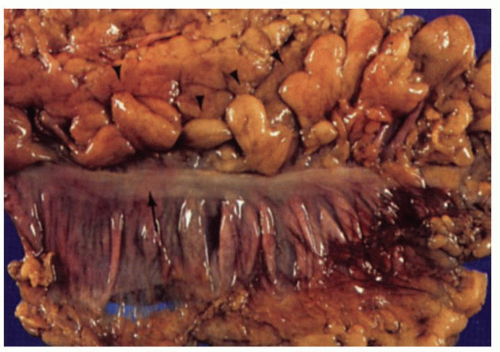 Figure 16-1. External surface of the large bowel showing the free taenia (arrow) and numerous appendices epiploicae (arrowheads). On either side of the tenia, the haustral folds can be seen. |
Peritonealized and Nonperitonealized Surfaces These are of practical importance because carcinomas of the large bowel in these regions can reach both peritonealized and nonperitonealized margins, which assume importance in the staging of carcinomas. Those reaching nonperitonealized margins being pT3, while those reaching (or breaching) peritonealized margins are pT4. The peritonealized margin is not a surgical margin, whereas the nonperitonealized margins often is the surgical resection margin. Inking of nonperitonealized margins in particular therefore should be undertaken to ensure that the relationship between the carcinoma and surgical margin is absolutely clear. At one time, peritonealized margins were called circumferential and the surgical nonperitonealized margins radial, but regrettably these have gradually become used interchangeably, so that now surgical margins are either nonperitonealized, mesenteric, proximal or distal, and nonsurgical margins are peritonealized.
The mesentery is variable along the length of the large bowel, and peritoneum surrounds it variably. Usually, the cecum is completely surrounded by peritoneum and partially mobile. The ascending colon, the hepatic and splenic flexures, and the descending colon are covered only anteriorly and therefore are relatively fixed. Carcinomas of these regions therefore have both peritonealized and nonperitonealized surface. The transverse and sigmoid colon have a mesentery and are therefore surrounded by peritoneum, except at their mesenteric attachments; these latter two regions are therefore particularly mobile. The rectum has no mesentery and rapidly loses its posterior, then its lateral, and finally its anterior covering of peritoneum by its middle third; the lower third is therefore subperitoneal. However, there is marked individual variability in the proportion of rectum covered by mesentery at any particular level. The rectum therefore has no “mesorectum,” simply surrounding tissue, so that the term mesorectal excision is a misnomer, albeit very practical. Of practical importance, and as suggested previously in this section, is that the presence of peritoneum on any surface allows assessment of the adequacy of local tumor excision by direct extension to its margin. In its absence, the irregular resected margins make adequate examination of these margins and comments on the adequacy of local excisions more difficult.1
Once removed from the body, all of these landmarks become almost useless. Any lesion within must be related to the only constant landmarks—the cecal pole, ICV, and pectinate line—or to the resected margins when only part of the large bowel is removed. Externally, the presence of a mesentery immediately suggests the transverse or sigmoid colon, while the flaring of the teniae denotes the rectum. It is therefore always wise for the surgeon to routinely insert a suture into one margin (e.g., proximal), so that the orientation of the specimen is always clear. The greater and lesser omenta are attached to the transverse colon in the immediate vicinity of an adjacent tenia.
Internal Appearance
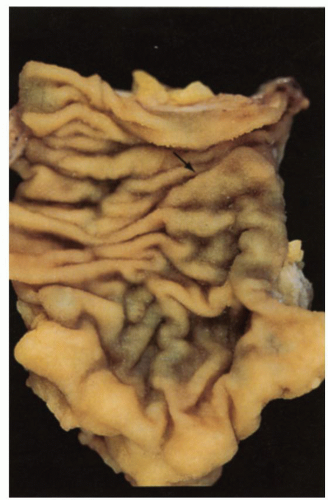
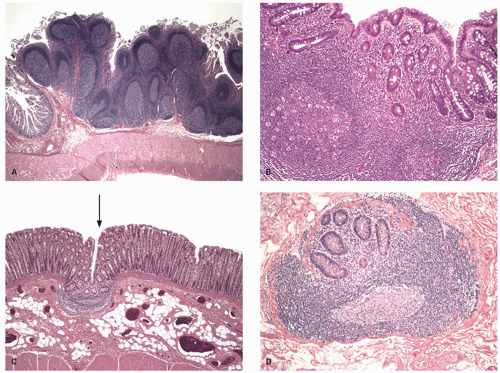
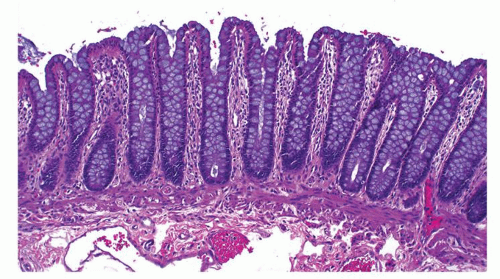
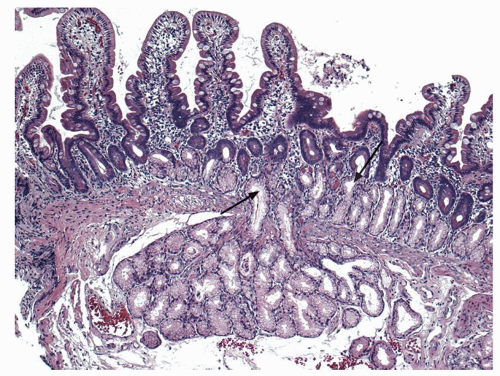
Small intestine. This is characterized by a series of mucosal folds, which vary from about 3 to 10 mm in height, running transversely around the bowel, although some form spirals running once or twice around the bowel. They are known as valvulae conniventes, although they are neither valves nor close off the lumen, folds of Kerckring or plicae circulares (Fig. 16-2). They gradually diminish through the distal jejunum and may be entirely absent from the distal ileum. These mucosal folds are studded at regular intervals with small nodules, which may vary from pinpoints to 3 mm in diameter and are the sites of underlying lymphoid nodules. The latter become more numerous distally, and in the terminal ileum are represented by larger antimesenteric Peyer’s patches (Fig. 16-2). These lymphoid follicles are very prominent in young individuals and begin
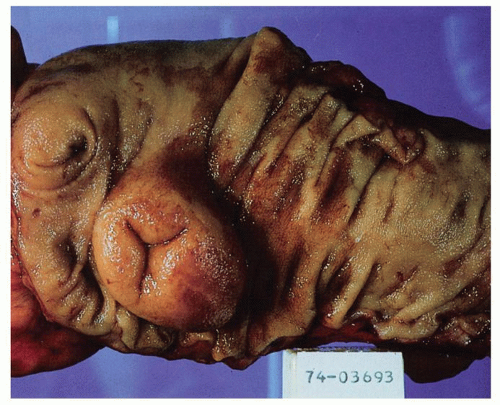
to atrophy in adulthood, so that by old age they may not be visible at all. They are 1 to 1.5 cm in width and 1 to 12 cm in length. They may be more prominent if they become hyperplastic as the result of an underlying disease process, in which historically they are best described, although there are no criteria for lymphoid hyperplasia. Small intestinal villi can usually be identified as pinpoint reflections of light in a freshly resected specimen but can be readily seen using magnification endoscopy. The ICV invaginates the medial wall of the large intestine, forming a large papilla-like structure, the orifice of which folds down to produce a stellate appearance when closed (see Fig. 16-3). The ICV behaves like a physiologic sphincter, preventing coloileal reflux. It consists of two lips, which taper to form folds that are part of the medial cecal wall. In some individuals, there is a very prominent accumulation of fat in the submucosa of the ICV region (Fig. 16-4).
to atrophy in adulthood, so that by old age they may not be visible at all. They are 1 to 1.5 cm in width and 1 to 12 cm in length. They may be more prominent if they become hyperplastic as the result of an underlying disease process, in which historically they are best described, although there are no criteria for lymphoid hyperplasia. Small intestinal villi can usually be identified as pinpoint reflections of light in a freshly resected specimen but can be readily seen using magnification endoscopy. The ICV invaginates the medial wall of the large intestine, forming a large papilla-like structure, the orifice of which folds down to produce a stellate appearance when closed (see Fig. 16-3). The ICV behaves like a physiologic sphincter, preventing coloileal reflux. It consists of two lips, which taper to form folds that are part of the medial cecal wall. In some individuals, there is a very prominent accumulation of fat in the submucosa of the ICV region (Fig. 16-4).
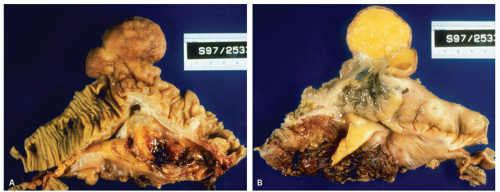 Figure 16-4. Extremely prominent ICV mimicking a tumor (A), that on cut sections shows fat often referred to as “lipoma of the ICV” (B). |
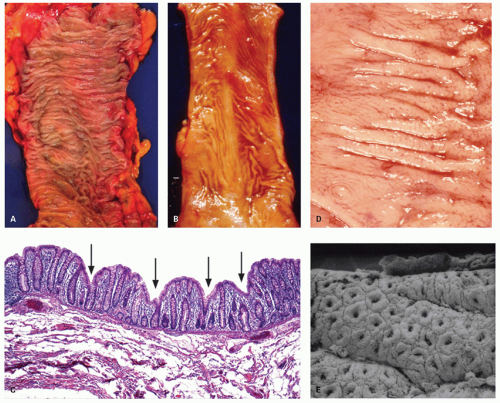
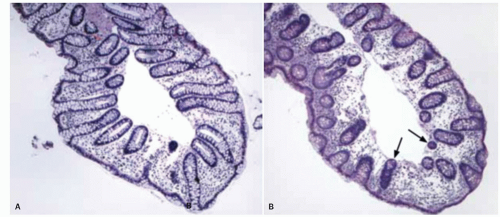
Large intestine. The internal surface of the large bowel is characterized by a series of haustra, which are saccules between the teniae. The boundaries of the haustra are also marked by semilunar folds (plicae semilunaris), which are analogous to the folds of Kerckring in the small intestine but are situated much farther apart and generally cover only part of the circumference (Fig. 16-5). The folds are more prominent proximally and may even disappear or become more irregularly arranged toward the rectum (Fig. 16-5B). The haustra are best seen on imaging studies or during endoscopy and tend to disappear once the colon is resected and opened. Careful inspection of the mucosa also reveals the presence of numerous innominate grooves running primarily transversely across the mucosa every few millimeters (Fig. 16-5D).2 Scanning electron microscopy suggests that between these grooves are smaller furrows into which crypts also open (Fig. 16-5D)3; histologically, these crypts tend to be intimately related focally to underlying lymphoid tissue (see below). These grooves also result in undulations of the mucosal surface forming so-called anthemic folds.
The appendiceal orifice is approximately 2.5 cm (range 1-3 cm) below the ICV, on the medial or posteriomedial cecal wall (Fig. 16-3). It is further described in Chapter 18. Lymphoid nodules can occasionally be identified, particularly in children, in whom they may be slightly dimpled (Fig. 16-5). The wall
thickness of the colon gradually varies from 0.2 to 0.5 cm and increases distally, being thickest in the sigmoid colon.
thickness of the colon gradually varies from 0.2 to 0.5 cm and increases distally, being thickest in the sigmoid colon.
Although the term rectum is derived from the Latin word meaning straight, because it is the straight part of the intestine (particularly in animals, in which the term was first coined), in practice it has three (and sometimes more) well-defined curves or valves (of Houston), the upper and lower ones curving to the right and the middle one to the left. The upper valve is about 4 cm below the rectosigmoid junction, the lower one about 3 cm above the dentate line. The middle valve is often the largest and is about 6 to 7 cm above the dentate line. It marks roughly the level of the peritoneal reflection and is approximately the limit of the examining finger.
Histology
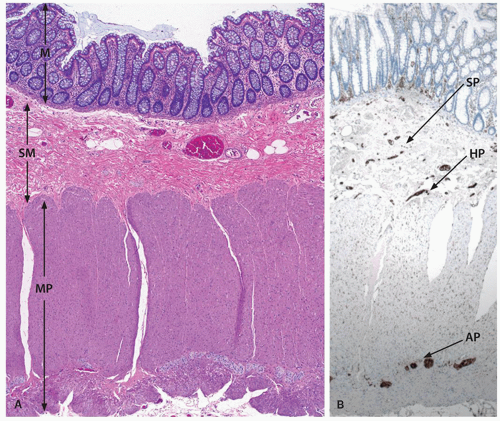
The layers of the small and large bowels are the mucosa, which includes the epithelium, lamina propria, and muscularis mucosae; the submucosa; the muscularis propria and subserosa or extra rectal fat; and the serosa.
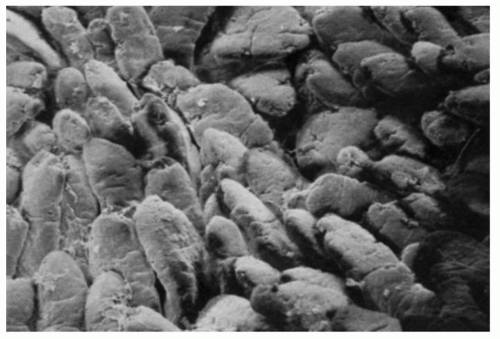 Figure 16-6. Scanning electron micrograph of jejunal villi. Around some of them, horizontal crevices can be seen which are also visible on light microscopy. |
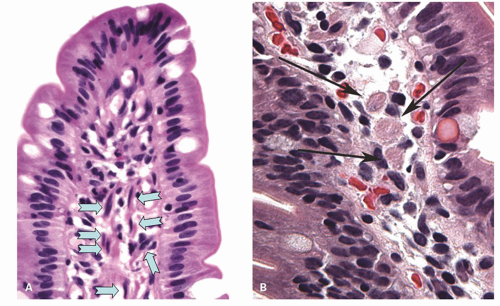
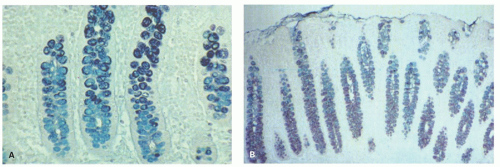
Small intestinal mucosa. The mucosa of the small intestine is lined by finger-like villi 0.5 to 1 mm in height. Scanning electron microscopy shows that the shape of the villi actually varies from being leaf-like to ridges in the duodenum to more finger-like as one moves distally in the jejunum and ileum (Fig. 16-6). The villous height varies in different regions of the small intestine, and in general the villous to crypt ratio varies from 3:1 to 5:1. In the duodenal bulb and descending duodenum, where Brunner’s glands are more abundant, the villi are shorter and stubbier. The villi are tallest in the distal and proximal jejunums and become progressively shorter from the jejunum to the terminal ileum (Fig. 16-7). They have a series of indentations (scalloping) along the sides of the villi, which are marked proximally but may disappear in the terminal ileum (Fig. 16-7). Throughout the small intestinal mucosa, the villi may be distorted by randomly scattered lymphoid follicles. These are much more numerous in the ileal mucosa.
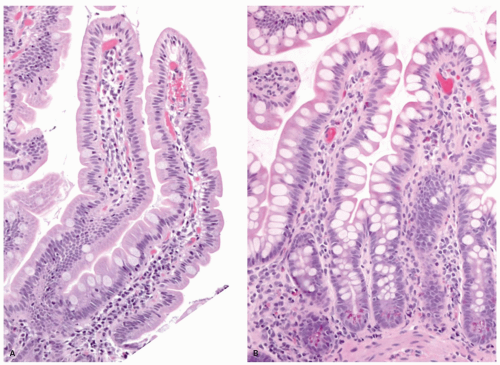 Figure 16-7. Proximal jejunal villi that are tall and leaf-like with scalloping of the borders (A). In comparison the ileal villi (B) appear shorter, more finger-like and contain more goblet cells. |
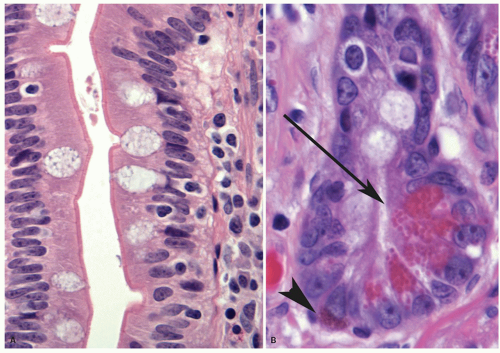
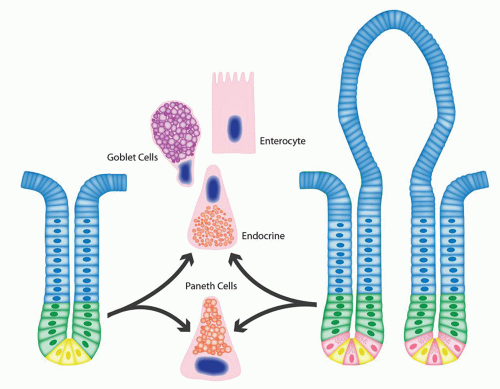
Epithelium Tall columnar absorptive cells (enterocytes) line the villi. Interspersed among them are goblet cells (Fig. 16-8), which sometimes appear to be increased in the second and third portions of the duodenum, and are unequivocally increased in relation to the number of columnar absorptive cells (enterocytes)
in the ileum. Indeed, a glance at the number of goblet cells is a very easy way of distinguishing proximal from distal small bowel (Fig. 16-7). The nuclei of the villous lining cells are regularly arranged at the bases of the cells. There are scattered intraepithelial lymphocytes in the villi, approximately 1 for every 5 to 10 epithelial cells (Fig. 16-8). Numerous lymphoid nodules are present either as isolated nodules or as aggregates that form Peyer’s patches in the ileum (Fig. 16-9A).
in the ileum. Indeed, a glance at the number of goblet cells is a very easy way of distinguishing proximal from distal small bowel (Fig. 16-7). The nuclei of the villous lining cells are regularly arranged at the bases of the cells. There are scattered intraepithelial lymphocytes in the villi, approximately 1 for every 5 to 10 epithelial cells (Fig. 16-8). Numerous lymphoid nodules are present either as isolated nodules or as aggregates that form Peyer’s patches in the ileum (Fig. 16-9A).
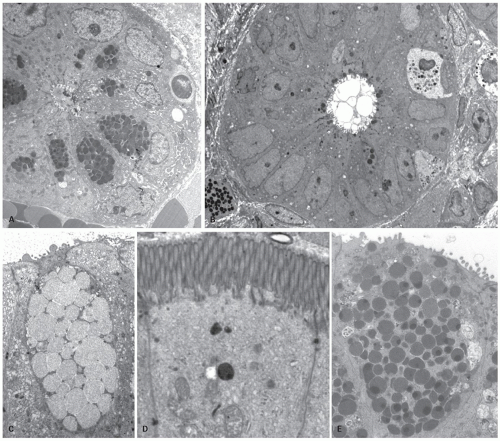
The predominant cells lining the villi are tall columnar absorptive enterocytes that have numerous microvilli on the luminal surface (Fig. 16-8). On the outer surface of the microvillous membrane is the glycocalyx, a glycoprotein coat just visible on routine hematoxylin and eosin (H&E) sections that can be highlighted by a diastase periodic acid-Schiff (D-PAS) stain or immunostains like carcinoembryonic antigen (CEA) or CD10 antibodies. It is better visualized on electron microscopy as a filamentous coating on the microvillous surface (Fig. 16-10D). This coat is produced by absorptive cells and is firmly adherent to the microvillous membrane. The microvilli of the columnar cells along with villi and mucosal folds (plica circularis) are responsible for providing the extensive surface area needed for absorption.4
The tubules that open at the bases of crypts constitute the crypts (crypts of Lieberkühn). This zone occupies approximately 20% of the total cell column that extends from the tips of the villi to the bases of the crypts. Multiple crypts merge into a vestibule at the base of a villus. In a two-dimensional plane, that is, in welloriented sections, one or two crypts are seen in continuity with some villi. The most abundant cells in the crypts are relatively undifferentiated cells that contain small cytoplasmic granules not usually visible at the light microscopic level. There is no distinct landmark that separates crypts from villi. In light microscopic sections, a band is seen approximately one-fifth of the way up from the muscularis mucosae, and this is often taken arbitrarily as the approximate crypt-villous junction. The columnar cells and goblet cells from the villous
surface are in continuity with the crypt epithelium. The crypts in addition harbor the undifferentiated stem cells and Paneth cells. Mitoses are seen near the crypt bases, with an average of one to two mitotic figures per welloriented crypt section. Paneth cells are present at the base of the crypts, and each crypt contains about 5 to 12 Paneth cells (Fig. 16-8B). The crypts also contains goblet cells, as well as cells that appear intermediate between undifferentiated crypt cells and goblet cells. The goblet cells that line the villi and crypts resemble those found elsewhere in the gut. Endocrine cells are also scattered throughout the crypt zone and are less frequently distributed along the sides of villi (Fig. 16-8B).
surface are in continuity with the crypt epithelium. The crypts in addition harbor the undifferentiated stem cells and Paneth cells. Mitoses are seen near the crypt bases, with an average of one to two mitotic figures per welloriented crypt section. Paneth cells are present at the base of the crypts, and each crypt contains about 5 to 12 Paneth cells (Fig. 16-8B). The crypts also contains goblet cells, as well as cells that appear intermediate between undifferentiated crypt cells and goblet cells. The goblet cells that line the villi and crypts resemble those found elsewhere in the gut. Endocrine cells are also scattered throughout the crypt zone and are less frequently distributed along the sides of villi (Fig. 16-8B).
Lamina Propria The lamina propria is the connective tissue core of the villi and extends from the villous tips to the muscularis mucosae. The most numerous cell types within the lamina propria are mononuclear cells, primarily plasma cells, and lymphocytes. Plasma cells are concentrated more in the intercrypt region. Other cell types in the lamina propria are much more sparsely distributed including eosinophils, macrophages, and mast cells. Normally neutrophils are rare. Longitudinal and branched eosinophilic fibers in the lamina propria of the villi represent smooth muscle cells that can be mistaken for macrophages (Fig. 16-11). Noncellular connective tissue elements, that is, collagen and elastin, are also present. Small blood vessels and fibroblasts and lacteals are in close contact with the basal laminae of the absorptive cells. The central lacteal is usually not easily seen unless pathologically enlarged or highlighted by immunohistochemical stains; this is probably the result of starvation before biopsy or resection. In the
duodenum and distal ileum, villi are normally stubbier and thus appear to contain increased lamina propria mononuclear (plasma cell and lymphocyte) cells compared to the mucosa from other regions of the small bowel.5 Numerous fine nerve twigs are present in the lamina propria, many of which pass through, or invest mast cells.
duodenum and distal ileum, villi are normally stubbier and thus appear to contain increased lamina propria mononuclear (plasma cell and lymphocyte) cells compared to the mucosa from other regions of the small bowel.5 Numerous fine nerve twigs are present in the lamina propria, many of which pass through, or invest mast cells.
ILEAL PIGMENT. Very frequently, a fine black pigment can be seen in macrophages in ileal biopsies, which is most prominent in the deep lamina propria or superficial submucosa, (Fig. 16-12) or within the macrophages in the region of Peyer’s patches. The pigment has been shown to contain calcium, phosphorus, titanium dioxide and, aluminum, and magnesium silicates. It is thought to be derived from diet, possibly from substances such as toothpaste which may contain titanium dioxide as a whitening agent; however, the mechanism speculative.6, 7 It appears to have no clinical significance, although when very prominent can cause diagnostic issues, especially if the patient has a history of melanoma. A similar pigment can be seen in the duodenum, often very prominently in some cases when it is referred to as melanosis duodeni or more appropriately psuedomelanosis duodeni, as the pigment is not melanin (Fig. 16-13). The pigment has
shown to be ferrous sulfide. Pseudomelanosis duodeni has been associated with chronic renal failure, use of antihypertensive drugs, iron pills, and prior mucosal hemorrhages.7, 8
shown to be ferrous sulfide. Pseudomelanosis duodeni has been associated with chronic renal failure, use of antihypertensive drugs, iron pills, and prior mucosal hemorrhages.7, 8
Ileocecal valve. The gross appearance of the aperture of the ICV is described above, and histologically also, many variations can occur.9 Usually a band of smooth muscle (Fig. 16-14A-C) that is contiguous with the muscularis propria of colon and ileum is seen running through the center of the valve and seems to end up joining the muscularis mucosae right at or very close to the junction of ileal and colonic mucosa (Fig. 16-14E).10 In children, its lips may be thickened because of the quantity of lymphoid tissue present, and the valve may appear everted in some individuals whereby the nodularity is visible from the colonic side (Fig. 16-14C). In adults, occasionally these nodules are biopsied as suspected colonic polyps. The adipose tissue in the substance of the valve varies among individuals and can be very prominent in some. This lipohyperplasia may mimic or be indistinguishable
from a true lipoma and is often even referred to as lipoma of the ICV (Fig. 16-4).
from a true lipoma and is often even referred to as lipoma of the ICV (Fig. 16-4).
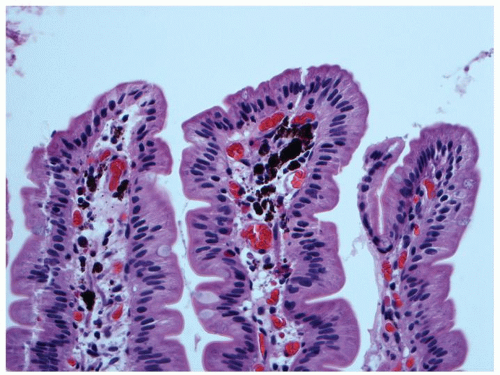 Figure 16-13. Pseudomelanosis duodeni showing accumulation of dark black pigment in the lamina propria macrophages in the duodenal mucosa. |
Large intestinal mucosa. Although the luminal surface of the colon is considered to be smooth, other than for the presence of haustra, careful examination of the surface mucosa reveals a series of surface irregularities that tend to go transversely around the bowel and are reminiscent of the small lines of fingerprints. These innominate grooves are readily seen using a hand lens or, even better, by low-power scanning electron microscopy (Fig. 16-5).3, 11 Similar but less well-defined lines join parallel irregularities to form small geographic areas. When this configuration is examined using ordinary light microscopy, the same changes are apparent, although there is some variation from patient to patient and even from area to area within the same patient. Stretching the mucosa
when pinning out resected specimens prior to fixation tends to make these changes less apparent and inevitably raises the question of whether they are normally present or represent an artifact. The fact that they are readily seen even with the naked eye if looked for, and are also visible both radiologically2 and even in biopsy material, strongly suggests that they are part of the normal anatomy. When viewed using the scanning electron microscope, it is apparent that the crypt openings are far better defined on the left colon than on the right.3 The reason for this is not clear.
when pinning out resected specimens prior to fixation tends to make these changes less apparent and inevitably raises the question of whether they are normally present or represent an artifact. The fact that they are readily seen even with the naked eye if looked for, and are also visible both radiologically2 and even in biopsy material, strongly suggests that they are part of the normal anatomy. When viewed using the scanning electron microscope, it is apparent that the crypt openings are far better defined on the left colon than on the right.3 The reason for this is not clear.
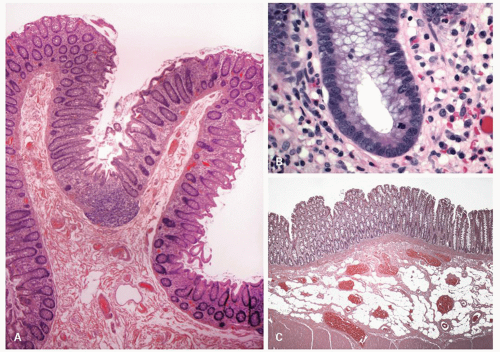 Figure 16-15. A: Overview showing colonic mucosal folds (plica semicircularis). Also note that at the bottom of the fold the crypts dip into a lymphoid aggregate similar to that seen in Fig. 16-9C. B: Detail of the base of colonic crypt showing lack of goblet cells, a population of undifferentiated cells in which the stem cells reside, and a mitotic figure. This is the zone that is occupied by the progenitor cells. C: Submucosal adipose tissue in colonic resection that is an incidental finding of no clinical significance. |
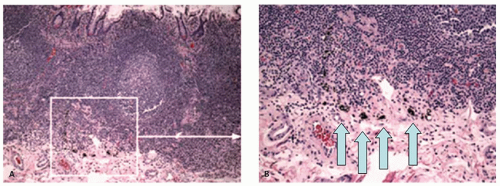
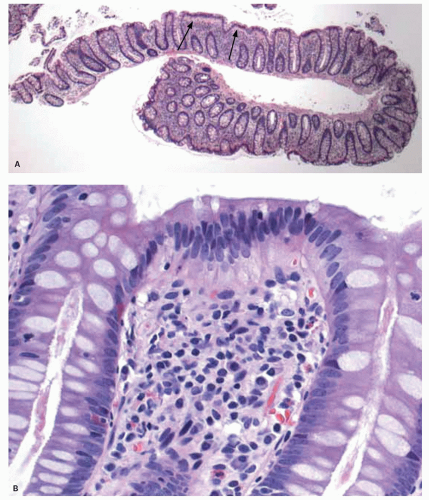
On light microscopy, it is apparent that crypts open both onto the surface of these ridges (anthemic folds) and into the crevices (innominate grooves) formed by them (Figs. 16-15 and 16-16). The function of the crypts in these grooves is likely as an antigen sampling apparatus analogous to the M-cells in the small intestine, and to increase the surface area, which may facilitate absorption, as does the presence of villi in the small intestine. However, careful examination by light microscopy, particularly if sections are not embedded perfectly, shows that at the base of many of these crypts is a lymphoid aggregate (Fig. 16-9C). Further examination reveals a change in the nature of the epithelium in the immediate vicinity of these aggregates from one that primarily contains goblet cells to one that appears to
consist entirely of absorptive cells (Fig. 16-9B). It is also apparent that there is an intimate relationship between the aggregate and the overlying follicle, with close apposition of one to the other but also with numerous lymphocytes passing through the epithelium, the majority of which are T cells (see subsequent discussion on lamina propria and immune system).12 Thus they are often referred to as lymphoglandular complexes rather than merely lymphoid aggregates.
consist entirely of absorptive cells (Fig. 16-9B). It is also apparent that there is an intimate relationship between the aggregate and the overlying follicle, with close apposition of one to the other but also with numerous lymphocytes passing through the epithelium, the majority of which are T cells (see subsequent discussion on lamina propria and immune system).12 Thus they are often referred to as lymphoglandular complexes rather than merely lymphoid aggregates.
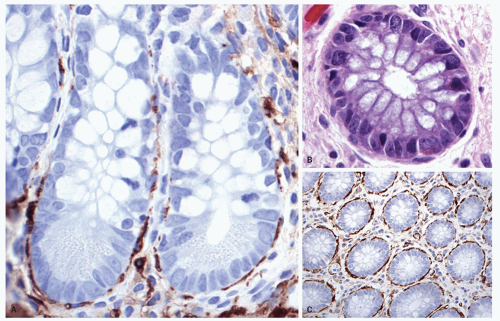
It is little less than amazing that a single layer of epithelium separates the potentially harmful contents of “bad outside world” in the bowel lumen from the internal milieu of the “body beautiful.” However, this protection is provided by a combination of protective mucus rich in secreted immunoglobulin A (IgA) that entraps bacteria, peristalsis that moves this layer of mucus, various antimicrobial secretions from the Paneth cells, and other component of the innate and adaptive immune system. This epithelium, besides forming a protective layer, performs a variety of tasks, reflected in the specialization into different cell types. The two predominant cells lining the colonic crypts are absorptive colonocytes (columnar) and goblet cells. The former are indistinct in the crypts where they are barely visible other than for their nuclei, while goblet cells are most conspicuous. The absorptive enterocytes on the other hand predominate on the surface where absorption occurs (Figs. 16-15 and 16-17) within the crypts. The absorptive colonocytes are tall columnar cells that show microvilli and a glycocalyx layer on the luminal surface similar to intestinal absorptive cells. They also contain special mucin vesicles that are different from goblet cell mucin. The apical portion of these cells fans out covering the tops of the adjacent goblet cells, such that only small part of the goblet cell opens into the lumen (Fig. 16-10). A common artifact seen in colonic biopsies at a low magnification is the appearance mimicking thickened subepithelial collagen layer (Fig. 16-17). On closer look this artifact is due to lighter staining of the infra-nuclear portion of the cytoplasm and the nucleus being pushed up in the middle of the cell. A fair number of endocrine cells are present through out the colon, and specialized microfold (M) cells are largely present in the areas of the lymphoid complexes. Paneth cells may normally be found on the right side, particularly in the vicinity of the ICV and the proximal ascending colon (Fig. 16-18). Paneth cells are generally absent beyond hepatic flexure, although rarely can be seen in the proximal transverse colon.
Specialized cells in the small and larger bowel epithelium
Paneth Cells Paneth cells have a very distinctive appearance with large bright eosinophilic granules clustered near the luminal aspect of the cells (Fig. 16-9B). Some cells that are more sparsely granulated and are intermediate between Paneth cells and goblet cells have also been recognized. Paneth cells have a relatively long life span of 20 days compared to 3 to 5 days for enterocytes. These cells have now been shown to play an important role in body’s defense against microbial infection and play an important role in innate and adaptive immune responses.13 They have been shown to contain a variety of antibacterial peptides and proteins such as lysozyme, alpha-defensins, secretory phopholipase-2, cryptdin-related sequence peptides, and angiogenin-4. Paneth cells secrete these antibacterial products at high levels in response to cholinergic stimuli and when exposed to bacterial antigens.13, 14 The release of these products into the crypt lumen is suggested to protect adjacent mitotically active crypt cells that are responsible for renewal of epithelium against enteric infections. The Paneth cells express a variety of toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-2 (NOD2), both of which play an important role in innate immune responses in GI tract.13 TLRs are transmembrane molecules, while NOD proteins are cytosolic and recognize microbial components after their invasion into the cell. These belong to a group of pattern recognition receptors that are germ line coded and do not require prior exposure to the pathogen. They recognize highly conserved motifs designated “pathogen-associated molecular patterns” (PAMPs), which are present in many microorganisms, but not in their hosts. These motifs are considered essential for the pathogenicity and survival of the microbes. Activation of these pattern recognition receptors (TLRs and NOD2) leads to release of various anti-microbial agents and chemokines that recruit other cellular component of the immune response. Thus, Paneth cells likely serve as guardian or protectors of the mucosa generally, but also the precious stems cells population located at the crypt bases. Mice transgenic for a human Paneth cell alpha-defensin, HD-5 are completely immune to infection and systemic disease from orally administered Salmonella enterica serovar typhimurium.15 In another experimental model, it has been shown that newborn mice are susceptible to infection by Shigella, but become resistant to infection by day 7 when the Paneth cells develop.16 Experimentally depleting the mice of Paneth cell induces the susceptibility to the same infection.16 These evidences provide a strong evidence for the role of Paneth cells in resistance to enteric infections. In humans, lack of Paneth cells in newborn infants has been linked to development of neonatal necrotizing enterocolitis (NEC).17 It is believed that lack of lysozyme may render these infants susceptible to bacterial translocation and subsequent sepsis. More interestingly, the Paneth cells have been now linked to inflammatory bowel disease (IBD), particularly Crohn’s disease.13 NOD2 recognizes a constituent
of peptidoglycan of bacterial cell wall called muramyl dipeptidase. Mutations in the NOD2 gene located on chromosome 16 have been associated with a group of patients with Crohn’s disease, whereby the defective gene product fails to recognize bacterial muramyl dipeptidase, leading to increased susceptibility of the intestinal mucosa to invasion by luminal bacteria and development of chronic inflammation. On the other hand, Paneth cell metaplasia in the colon in various chronic inflammatory conditions including IBD may be an attempt to protect the damaged epithelium from luminal microbes. Decrease in Paneth cells has also been reported with development of complications in celiac sprue (refractory celiac sprue and ulcerative jejunoileitis/enterpathy-associated T-cell lymphoma).18
of peptidoglycan of bacterial cell wall called muramyl dipeptidase. Mutations in the NOD2 gene located on chromosome 16 have been associated with a group of patients with Crohn’s disease, whereby the defective gene product fails to recognize bacterial muramyl dipeptidase, leading to increased susceptibility of the intestinal mucosa to invasion by luminal bacteria and development of chronic inflammation. On the other hand, Paneth cell metaplasia in the colon in various chronic inflammatory conditions including IBD may be an attempt to protect the damaged epithelium from luminal microbes. Decrease in Paneth cells has also been reported with development of complications in celiac sprue (refractory celiac sprue and ulcerative jejunoileitis/enterpathy-associated T-cell lymphoma).18
Endocrine Cells Endocrine cells are also scattered throughout the crypt zone in both small and large bowel, and are less frequently distributed along the sides of villi. They can be demonstrated with a variety
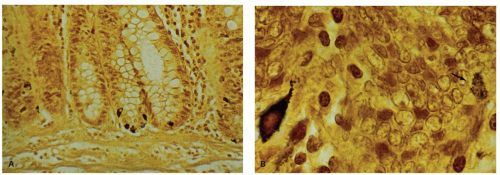
of techniques including silver stains, IHC markers and electron microscopy. Morphologically, two types are recognized. One type has inconspicuous granules on H&E sections, often communicates with the lumen, and has a basal nucleus. These cells cannot be easily identified on H&E sections. The other type is the enterochromaffin cell with its characteristic subnuclear orange granules that are either pyramidal or spindle shaped on H&E sections (Fig. 16-8). In the small intestine, more gut endocrine cells are located in the upper small intestine than in the lower portion, and rare endocrine cells can sometimes be found apparently isolated in the lamina propria. The endocrine cells throughout the intestinal tract can be easily highlighted using either silver stains or immunostains. The silver stains identify two types of cells. The most easily demonstrable of these are argentaffin cells that stain the serotonin-producing enterochromaffin cells. The name argentaffin implies that they will cause the deposition of silver from ammoniacal silver nitrate onto themselves in the absence of another reducing agent; the Masson-Fontana stain has precisely these characteristics. By contrast, other endocrine cells require a reducing agent for silver to be deposited—a so-called argyrophil stain; examples include the Grimelius, and Chirukian and Schenk stains (Fig. 16-19). At least three other types of argyrophil cells containing densecore neurosecretory granules can be demonstrated.19
Immunohistochemical stains identify at least 30 different types of endocrine cells distributed throughout the GI tract. Immunostains, because of their higher specificity and sensitivity, are superior to the silver stains, and have replaced them in practice for the identification of endocrine cells. The various cells identified in the intestines include cells that contain serotonin, somatostatin, cholecystokinin, secretin, gastric inhibitory peptide, enteroglucagon, substance P, neurotensin, vasoactive intestinal polypeptide (VIP), enteroglucagon, substance P, L cells, and motilin. Regional differences in the distribution of these cells exist. It is interesting that adenomas and adenocarcinomas of the large bowel not infrequently contain Paneth cells and endocrine cells. However, so-called goblet cell carcinoids (usually appendiceal) contain goblet cells, neurosecretory cells, and rarely Paneth cells, while true carcinoid tumors are almost entirely endocrine. This likely suggests origin of the adenomas and carcinomas from a more primitive cell type compared to endocrine tumors. It is interesting that in the midgut (jejunum, ileum, and proximal large bowel), virtually all endocrine tumors are of the enterochromaffin cells type. Distal to this in the large bowel, L-cell tumors occur, and the distribution of the tumors mirrors the distribution of these cells in the large bowel, which predominate in the rectum, although these tumors are also common in the appendix. This admixture of neurosecretory cells with all other cell lines in neoplasms, together with the elegant work of Le Douarin,20 strongly indicates that these endocrine cells in the GI tract are of epithelial (endodermal) and not neural crest origin.
of techniques including silver stains, IHC markers and electron microscopy. Morphologically, two types are recognized. One type has inconspicuous granules on H&E sections, often communicates with the lumen, and has a basal nucleus. These cells cannot be easily identified on H&E sections. The other type is the enterochromaffin cell with its characteristic subnuclear orange granules that are either pyramidal or spindle shaped on H&E sections (Fig. 16-8). In the small intestine, more gut endocrine cells are located in the upper small intestine than in the lower portion, and rare endocrine cells can sometimes be found apparently isolated in the lamina propria. The endocrine cells throughout the intestinal tract can be easily highlighted using either silver stains or immunostains. The silver stains identify two types of cells. The most easily demonstrable of these are argentaffin cells that stain the serotonin-producing enterochromaffin cells. The name argentaffin implies that they will cause the deposition of silver from ammoniacal silver nitrate onto themselves in the absence of another reducing agent; the Masson-Fontana stain has precisely these characteristics. By contrast, other endocrine cells require a reducing agent for silver to be deposited—a so-called argyrophil stain; examples include the Grimelius, and Chirukian and Schenk stains (Fig. 16-19). At least three other types of argyrophil cells containing densecore neurosecretory granules can be demonstrated.19
Immunohistochemical stains identify at least 30 different types of endocrine cells distributed throughout the GI tract. Immunostains, because of their higher specificity and sensitivity, are superior to the silver stains, and have replaced them in practice for the identification of endocrine cells. The various cells identified in the intestines include cells that contain serotonin, somatostatin, cholecystokinin, secretin, gastric inhibitory peptide, enteroglucagon, substance P, neurotensin, vasoactive intestinal polypeptide (VIP), enteroglucagon, substance P, L cells, and motilin. Regional differences in the distribution of these cells exist. It is interesting that adenomas and adenocarcinomas of the large bowel not infrequently contain Paneth cells and endocrine cells. However, so-called goblet cell carcinoids (usually appendiceal) contain goblet cells, neurosecretory cells, and rarely Paneth cells, while true carcinoid tumors are almost entirely endocrine. This likely suggests origin of the adenomas and carcinomas from a more primitive cell type compared to endocrine tumors. It is interesting that in the midgut (jejunum, ileum, and proximal large bowel), virtually all endocrine tumors are of the enterochromaffin cells type. Distal to this in the large bowel, L-cell tumors occur, and the distribution of the tumors mirrors the distribution of these cells in the large bowel, which predominate in the rectum, although these tumors are also common in the appendix. This admixture of neurosecretory cells with all other cell lines in neoplasms, together with the elegant work of Le Douarin,20 strongly indicates that these endocrine cells in the GI tract are of epithelial (endodermal) and not neural crest origin.
Goblet Cells The cells are named so because of their shape that resembles wine goblets. The mucous globules in the goblet cell cause the luminal aspect of the cytoplasm to balloon, and the nucleus appears cramped and scalloped at the base of the cell. In animals, variants of columnar absorptive cells, but with shortened microvilli that appear preferentially to attract bacteria, have been described as cup cells while other cells have microvilli extending beyond those of normal enterocytes, so-called tuft cells.21 They have yet to be adequately documented in man. The goblet cells strongly stain with Muc2, a mucin that has a protective affect against luminal infectious organisms.22, 23, 24 It has been calculated that goblet cells make up 20% to 30% of the cells in the right colon and 40% to 50% of those in the left colon. There may be a gradual increase in the number of goblet cells from the right colon to the left, but this pattern is rarely clear enough to allow one to predict reliably from which part of the large bowel a particular biopsy specimen was taken. Nevertheless, scanning electron microscopy and mucin staining (Fig. 16-20) also suggest that there are differences between both sides of the large bowel. There is also a change in the quality of mucus on both sides of the colon, particularly the ratio of sulfated to nonsulfated mucin,25 and lack of sulfated mucin has been correlated with possible premalignant changes.26, 27, 28 Alterations in mucin structures in cancer have many biologic and pathologic consequences.29 They can influence the growth and survival of the caner cells, their ability to invade and metastasize, and their interactions with lectins and cell-surface receptors or cells of the immune system. Mucins also interact with adhesion molecules of cells and can block cell-to-cell adhesion mediated by integrins and E-cadherin. Mucins can also inhibit cell lysis by natural killer cells and interactions with cytotoxic lymphocytes.
The mucins covering the surface not only provide a physical barrier, but allow adherence to many commensal bacteria that competitively inhibit binding of the pathogenetic organisms. The secretion of mucins can be modified by activation of pathogen recognition receptors (PRR) and TLR present on the epithelial cells.22
Microfold (M) Cells M cells are so called because of their surface microfolds (microfold cells) or thin membranous cytoplasm (membrane cell) rather than microvilli. The cells are membrane-like (and also macrophage-like), rather than the adjacent tall columnar epithelium. They are not readily visualized by light microscopy and lack a specific immunohistochemical marker for easy identification, although in rabbits they express vimentin that helps in differentiation from adjacent enterocytes.30 They have been largely characterized by electron microscopy.4, 31
These cells typically have poorly organized brush border with fewer microvilli and lack a glycocalyx layer.32 Many lymphocytes can be seen indenting the cell cytoplasm, almost appearing as if they are within the cell cytoplasm. In many places, a thin sheet of cytoplasm is all that separated the lymphocytes from the lumen; M cells transport soluble and particulate antigens from the lumen to the cells in the underlying lymphoid follicle and can therefore be regarded as the component of gut-associated lymphoid tissue (GALT) epithelial cells, or more generically called mucosaassociated lymphoid tissue (MALT); however, they also transport macromolecules in the opposite direction.33 They transport the luminal antigens, various macromolecules, bacteria, or virus to the lamina propria to be exposed to the immune system. Whether these cells can also present antigens to the immune system similar to dendritic cells is still unclear. Large particles and bacteria are internalized via phagocytosis, while viruses and adherent particles by endocytosis via clathrin-coated vesicles.32 Surprisingly, they are also characterized by the absence of secretory component (SC) expression in the animal model34; they therefore appear to be unable to secrete IgA, which may, at least partially, explain the sensitivity of these cells to a variety of organisms and their toxins. While most of the M cells are present in the vicinity of the lymphoid follicles, they can be seen in the surface epithelium of villi as well as elsewhere away from the lymphoid tissue.35 It is still unclear whether the M cells are derived from transformation of adjacent enterocytes, although recently it has been suggested that they are possibly derived from a separate pathway from the progenitor cells.30
Stem cells and kinetics. The mature cells near the tip of the villi or surface of the colon die from apoptosis and are continuously either shed into the lumen, or, especially in the right side of the large bowel, undergo apoptosis. As the cells die at the surface, transient gaps or holes are created that are immediately sealed off by adjacent cells. Apoptotic nuclei are often ingested by macrophages immediately beneath the surface epithelium that may be lightly pigmented. Cell replication within small and large bowel occurs in the crypts and with a turnover time of about 3 to 6 days. Due to this high turnover of cells in the small and large bowel, it is not surprising that the progenitor cells constitute an important cell population in the crypts. Their role in cancer development has further ignited a significant interest in them.36, 37 Most of the data come from experiments in mice, and ingenious labeling studies have helped to track the pattern of cell proliferation and movement of cells in the epithelium, especially in the small bowel.38, 39 It has been fairly clear for many years that the stem cells are located at the base of the crypts although the precise location and identification has been debated. The most actively dividing cells, as seen with long-term tritiated thymidine incorporation, bromodeoxyuridine (BRDU) incorporation or proliferation markers (Ki67) (Fig. 16-21), are seen at the sides of the crypts just above the level of Paneth cells and have a feedback loop with the adjacent Paneth cells (Fig. 16-22). These are called transit amplifying cells and undergo rapid proliferation producing about 300 cells per crypt per day that eventually differentiate into four major intestinal cell types-absorptive enterocytes, goblet cells, endocrine cells, and Paneth cells. However, it appears that the stem cells are fewer and reside at the deepest part of the crypt base and are surrounded by Paneth cells on each side.37 These have been called crypt base columnar cells (CBC).40 It is now shown that these stem cells express Lgr5/Gpr49 gene, which encodes an orphan G protein-coupled receptor and appears to be reliable stem cell marker in the intestine. The stem cells are believed to undergo asymmetric division by which they produce one stem cell (self-renewal) and another committed cell that become committed progenitor cells and undergo further divisions to differentiate into various other intestinal cells types. Under normal conditions, Ki67 stain shows only occasional positive nuclei at cyrpt bases in the stem cells zone (Fig. 16-21C); however, under stress or conditions that require rapid cell renewal, the zone expands.
The cells arising from these progenitor cells migrate upward in the crypts and eventually on to the luminal surface or surface of the villi, a process that takes approximately 3 to 6 days in man. As the cells move upward, they differentiate into absorptive cells, goblet cells, M cells, and endocrine cells. The cells migrating downward into the crypts differentiate into Paneth cells, which move to the bottom of the crypts. The differentiation of the cells into various cell types is complex and involves NOTCH and Wnt signaling pathways. The progeny from a single stem cell at the bottom of a crypt represents a clone of cells that moves up along the crypt in the form of a ribbon. Sometimes the entire crypt may consist of a single clone.
Pericrypt fibroblast sheath. Surrounding the crypts of the small and large bowel is the pericrypt fibroblast
sheath, which is closely apposed to the epithelial cells (Fig. 16-23). In some biopsies, this is relatively prominent, in others virtually invisible. A complex epithelial-mesenchymal interaction exists between these pericryptal fibroblasts and the crypt epithelium. The fibroblasts have been thought to proliferate at the same rate as the crypt epithelium and to migrate with the epithelium.41 However, some studies suggest that the fibroblast may in fact have a much slower turnover time, which would necessitate the epithelial cells “sliding” up along the sheath.42 This fibroblastic sheath is absent around the gastric glands, and it has been shown in a study that with intestinal metaplasia in the stomach with the development of intestinal type epithelium, a pericryptal fibroblastic sheath also develops.43 While it may therefore be better to think of the crypts as an integral epithelial-fibroblast complex, this concept poses problems. If both crypts and fibroblasts migrate at the same rate, what happens to the fibroblasts when they reach the epithelium immediately beneath the lumen? Clearly, they do not simply pile up and form tumor masses; possibly they escape into the lumen by passing through or between surface epithelial cells. Presumably, they either die immediately and are phagocytosed by local macrophages, transform into another cell population (possibly subepithelial macrophages), migrate elsewhere, or are simply not formed in the same numbers as epithelial cells.
sheath, which is closely apposed to the epithelial cells (Fig. 16-23). In some biopsies, this is relatively prominent, in others virtually invisible. A complex epithelial-mesenchymal interaction exists between these pericryptal fibroblasts and the crypt epithelium. The fibroblasts have been thought to proliferate at the same rate as the crypt epithelium and to migrate with the epithelium.41 However, some studies suggest that the fibroblast may in fact have a much slower turnover time, which would necessitate the epithelial cells “sliding” up along the sheath.42 This fibroblastic sheath is absent around the gastric glands, and it has been shown in a study that with intestinal metaplasia in the stomach with the development of intestinal type epithelium, a pericryptal fibroblastic sheath also develops.43 While it may therefore be better to think of the crypts as an integral epithelial-fibroblast complex, this concept poses problems. If both crypts and fibroblasts migrate at the same rate, what happens to the fibroblasts when they reach the epithelium immediately beneath the lumen? Clearly, they do not simply pile up and form tumor masses; possibly they escape into the lumen by passing through or between surface epithelial cells. Presumably, they either die immediately and are phagocytosed by local macrophages, transform into another cell population (possibly subepithelial macrophages), migrate elsewhere, or are simply not formed in the same numbers as epithelial cells.
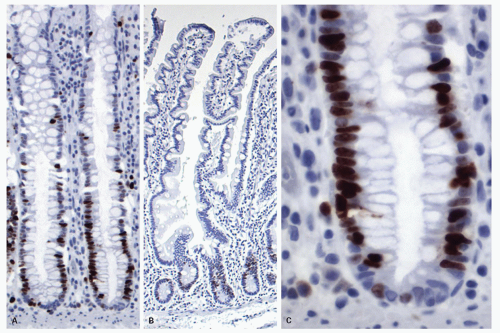 Figure 16-21. Ki-67 staining to show aggregation of positive nuclei at the crypt bases showing the location of the proliferative compartment (progenitor cells) in the colon (A) and small intestine. The most intensely staining area is not exactly at the base but slightly higher on each side of the base, just above the location of Paneth cells in the small bowel, and represents the transit activating (TA) cells. B: Detail of a colonic crypt showing that the deepest part of the crypts contains only rare positive nuclei and possibly represent quiescent intestinal stem cells. C: Compare the staining with the schematic diagram showing various progenitor cell populations in small and large bowel (Fig. 16-22). |
However, if their turnover time is indeed slow, absorption of all substances must be accomplished by epithelial cells (presumably throughout the entire gut) at a time when they are actively migrating relative to the immediately adjacent cell population—an intriguing thought. On the contrary, the epithelial turnover is slowed in hyperplastic/serrated family of polyps, and it is interesting that there is often associated fibroblastic growth in the adjacent lamina propria forming so-called fibroblastic polyps in about one-third of cases.44, 45 Whether this represents lack of switching-off of the growth of the fibroblastic sheath or slow and continued growth is another intriguing thought that needs further investigation. The pericrypt fibroblast sheath and the adjacent collagen table are thickened in a variety of conditions, including collagenous colitis and hyperplastic polyps, and possibly different mechanisms are involved.
Lamina propria and immune system. The lamina propria of the bowel is also a neglected organ, usually being considered as an accumulation of chronic inflammatory cells that tends to increase in some
inflammatory conditions. Nevertheless, the lamina propria is highly organized structurally. Immediately beneath the luminal epithelium and in the large bowel extending down up to a quarter of the way to the muscularis mucosae (also an integral part of the mucosa) is frequently a layer of macrophages. This layer is readily demonstrated with the PAS stain and which often contain nuclear debris. This is usually more apparent in the large intestine. Beneath it and continuing close to the muscularis mucosae is a layer consisting largely of plasma cells in which occasional histiocytes, eosinophils, rarely, a neutrophil, lymphocytes, and sometimes surprisingly large numbers of mast cells are located. However, in the large bowel, except for the region of the ICV, plasma cells virtually never reach the basal 1/4th of the mucosa; in this region, are primarily lymphocytes, often eosinophils, and occasionally mast cells and histiocytes.46 The appearance of more than occasional plasma cells usually indicates long-standing chronic inflammation such as that seen in IBD. If quantified, excluding the muscularis mucosae, the lamina propria constitutes 54% (and the epithelium 46%) of the mucosa. Point counts reveal that plasma cells make up 47% of the cells in the lamina propria, mesenchymal cells 20%, and macrophages, lymphocytes, and eosinophils about 10% each. Rarely are neutrophils present and, other than an occasional cell, are abnormal.47
inflammatory conditions. Nevertheless, the lamina propria is highly organized structurally. Immediately beneath the luminal epithelium and in the large bowel extending down up to a quarter of the way to the muscularis mucosae (also an integral part of the mucosa) is frequently a layer of macrophages. This layer is readily demonstrated with the PAS stain and which often contain nuclear debris. This is usually more apparent in the large intestine. Beneath it and continuing close to the muscularis mucosae is a layer consisting largely of plasma cells in which occasional histiocytes, eosinophils, rarely, a neutrophil, lymphocytes, and sometimes surprisingly large numbers of mast cells are located. However, in the large bowel, except for the region of the ICV, plasma cells virtually never reach the basal 1/4th of the mucosa; in this region, are primarily lymphocytes, often eosinophils, and occasionally mast cells and histiocytes.46 The appearance of more than occasional plasma cells usually indicates long-standing chronic inflammation such as that seen in IBD. If quantified, excluding the muscularis mucosae, the lamina propria constitutes 54% (and the epithelium 46%) of the mucosa. Point counts reveal that plasma cells make up 47% of the cells in the lamina propria, mesenchymal cells 20%, and macrophages, lymphocytes, and eosinophils about 10% each. Rarely are neutrophils present and, other than an occasional cell, are abnormal.47
The number of inflammatory cells in the lamina propria varies between the right and left sides of the colon. For example, the right side of the colon, especially cecum or close to the ICV, the mucosa contains increased number of lamina propria lymphocytes and plasma cells and normally appears somewhat “inflamed” and cause problems in interpretation even for the most experienced of pathologists (Fig. 16-18).48 Anecdotally, this also seems to be age related, increasing with age, so that inflammation that may be accepted as normal in older patients, may be pathologic in children. Occasional Paneth cells are common in the right colon, and sometimes the presence of eosinophils and even neutrophils may further increase this confusion. In practice such mild inflammatory changes in the right side of the colon are not uncommon in asymptomatic individuals and likely represent a normal variation.
A common practice of gastroenterologists is to put all the colonic biopsies from various segments of the colon during random sampling in one container, which may lead to an erroneous diagnosis by the pathologists of focal/patchy inflammation of the colon. Ideally biopsies should be placed in separate containers by site, although financial constraints sometimes do not allow this, and what one can do is be at least selective. Knowing whether the rectum is involved or not is critical for the diagnosis of ulcerative colitis, so putting all biopsies from, for example, the left colon, in one container, denies the opportunity of proper evaluation. The pathologist should also be aware of these pitfalls.
Abutting onto the muscularis mucosae are the numerous lymphoid aggregates mentioned above. These tend to straddle the muscularis mucosae and actually disrupt it. The cellular immune system of the large bowel therefore consists of luminal epithelium, a layer of macrophages, a deeper layer consisting largely of plasma cells, and finally, numerous lymphoid aggregates that have access to the lumen through the infoldings of the surface.
Plasma cells in the colon consist largely of IgA containing cells, which constitute about 90% of all plasma cells their number being only slightly less in the small bowel. The remaining cells are largely IgM- and IgG-containing cells.
The epithelium has numerous absorptive functions, which are carried out primarily in the microvilli or on the surface epithelium, and also a secretory function, which is primarily carried out in crypts. In addition, except for the dome epithelium overlying lymphoid nodules, the remaining epithelium secretes large amounts of IgA into the lumen, amounting to about 3 gm of immunoglobulin daily, more than the entire amount of IgG produced by the rest of the body on a daily basis. Local plasma cells in the lamina propria secrete IgA (and IgM) in a polymeric form attached to J-protein, for which epithelial cells have receptors in the form of SC on their membranes. The entire secretory piece-J chain-Ig complex is internalized, passes across the cell, and is resecreted into the apical surface of epithelial cells (Fig. 16-24). Interactions between activated leukocytes such as lymphocytes and macrophages may upregulate the SC-dependent immunoglobulin transport mechanism, and this may be of immense significance in disease.49
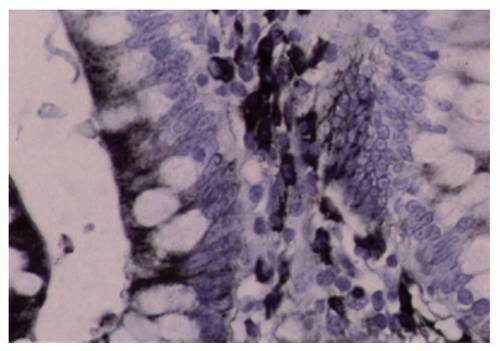 Figure 16-24. IgA immunoreactivity in the small and large bowel is found in the majority of lamina propria plasma cells and in the apices of the epithelial cells. |
In inflammatory disease, the ratios change dramatically, with an increase particularly in IgG-containing cells. In various immunodeficiency diseases, plasma cells may be markedly reduced or even absent (see Chapter 3).
Throughout the small and large bowel there are definite lymphoepithelial complexes that represent at least part of the cellular immunologic system of the bowel, also called MALT. The organization of this lymphoid tissue is discussed in more detail in Chapter 4. Indeed, it is apparent that not only are there T and B areas around normal lymphoid aggregates, but a variety of helper and suppressor cells are present—many being class specific—that modify each other’s action. Many intraepithelial T cells present. Also of interest is the fact that mucus secretion by goblet cells may be stimulated by immune complexes50 and by antigens in animals that have been orally immunized51 so that T cells may modify mucus secretion.32, 52
For a more detailed discussion of the organization of gut immune system, refer to Chapters 3 and 4.
Muscularis mucosae. This is a thin structure, often less than 10 cells thick, but in which two layers of fibers can sometimes be visualized, thereby mimicking the muscularis propria. It is the basal part of the mucosa and is permeated by nerves, blood vessels, and lymphatics.
Submucosa. The submucosa consists primarily of connective tissue, blood vessels, nerves, and lymphatics.37 Generally, the submucosa is devoid of adipose tissue. However, presence of diffuse adipose tissue (fatty metaplasia) can be seen not infrequently without any associated disease and may even represent an age-related phenomenon.
The ganglion cells and nerve fibers of the submucosal plexus (Meissner’s and Henle’s) are located here. Other isolated cells are very sparsely distributed and include most of the cells described in the lamina propria; frequently mast cells are the predominant cell type. In the ileum, aggregates of lymphoid follicles, Peyer’s patches, may occupy both the mucosa and the submucosa and may disrupt the muscularis mucosae. In the more proximal small intestine, lymphoid follicles are more sparsely distributed, and may be found in the basal portion of the mucosa or in the submucosa. In colon, the lymphoid follicles extend in the submucosa and sometimes along with crypts which on tangential sections could be mistaken for infiltrating glands (Fig. 16-9D).
Brunner’s glands are mucus-secreting glands primarily confined to the submucosa of the duodenal bulb and the second portion of the duodenum, but sometimes straying beyond the ligament of Trietz. In H&E-stained sections, they are clear-staining, similar to antral glands (Fig. 16-25). Sometimes they contain nests of cells that appear more eosinophilic and granular, bearing a resemblance to hybrid Paneth cells; like Paneth cells, these cells are also lysozyme positive and contain epidermal growth factor (see Chapter 12). In the duodenal bulb, Brunner’s glands commonly occupy the basal part of the mucosa, connect with the overlying crypts, and pierce the muscularis mucosae to the submucosa below. In the duodenal bulb, their presence may result in a localized or diffuse finely nodular appearance at endoscopy. In the second portion of the duodenum and beyond, Brunner’s glands are located primarily in the submucosa. They taper off in density in the third and fourth portions of the duodenum. The ducts of these glands are virtually indistinguishable from superficial gastric mucinproducing cells.
Muscularis propria. The muscularis propria of small and large intestines is organized into inner circular muscle and outer longitudinal muscle layers. The outer longitudinal layer in the colon forms localized thick bands called the taenia coli (see above). The muscularis propria of the colon continues into the anal canal (see Chapter 22).
Nerve supply, myenteric plexi, and interstitial cells of Cajal. Normal bowel motility results from a complex interplay between various components of the neuromuscular apparatus of the GI tract, which includes smooth muscle of muscularis mucosae and muscularis propria, interstitial cells of Cajal (ICCs), the intrinsic and extrinsic nerve supply, and various neuroendocrine peptides. The ICCs generate the slow waves of peristalsis, while the muscle fibers provide the contractile
force. The extrinsic nervous system and neuroendocrine peptides provide the external control for this movement (peristalsis), while the intrinsic nerves help to coordinate all these activities. Abnormality in any of these components may result in bowel dysmotility.
force. The extrinsic nervous system and neuroendocrine peptides provide the external control for this movement (peristalsis), while the intrinsic nerves help to coordinate all these activities. Abnormality in any of these components may result in bowel dysmotility.
Nerve Supply and Myenteric Plexus The nerve supply to the bowel is from the sympathetic and parasympathetic systems. Most of the sympathetic fibers originate from the prevertebral ganglia. These fibers generally follow the branches of the superior and inferior mesenteric arteries. The large celiac branch of the posterior trunk of the vagus nerve supplies the parasympathetic innervation to the bowel. They culminate in the plexi of the bowel.
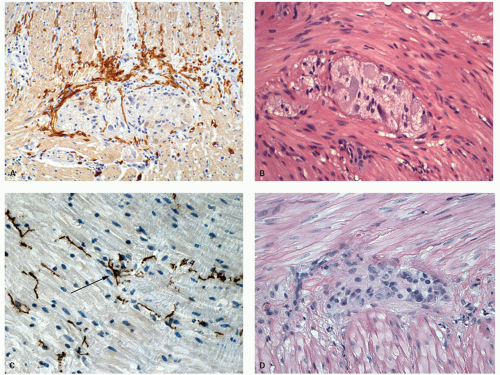
Major neural plexi are located in the mucosa, submucosa, and muscularis propria. Of these, the submucosal plexus (Meissner’s plexus), deep submucosal plexus (Henle’s plexus), and intermuscular myenteric plexus (Auerbach’s plexus) contain a collection of ganglion cells accompanied by numerous Schwann (glial) cells (Fig. 16-26). Occasional ganglion cells can be sometimes found in the deep lamina propria in the absence of any disease, however, their presence in mucosa in more numbers, or clusters or higher up in the lamina propria should alert one to look for associated disorders (IBD, ganglioneuroma associated with MEN2b syndrome, von Reckinhausen’s disease) (see Chapter 8).
The mucosal plexus is not only partly pericryptal and subepithelial but also distributed diffusely. They may be ultimately related to inflammatory cells, particularly mast cells and eosinophils as well as crypts.53, 54 In infants, in a standard section, about one nerve fiber can be found between every three to five crypts. In adults, these nerve fibers are more plentiful. These consist of argyrophilic and nonargyrophilic axons (see Chapter 6). The nerve fibers in the mucosa can be stained with various IHC neural markers S100, neuron specific enolase or PGP9.5, while VIP containing nerves also appear plentiful. The unmyelinated fibers are normally present only in the deepest part of the lamina propria just at the upper levels of muscularis propria, and can be highlighted by histochemical staining for acetylcholinesterase on frozen sections. In the distal 2 cm of the rectum at the anorectal junction, there may be a paucity of ganglion cells normally with hyperplasia of the submucosal
and mucosal plexi, which can mimic the changes seen in Hirschsprung’s disease.55
and mucosal plexi, which can mimic the changes seen in Hirschsprung’s disease.55
The myenteric (Auerbach’s) plexus is the most easily recognized of all the neural plexi, consisting of collection of ganglion cells (generally 3-5) between the circular and longitudinal layers of the muscularis propria (Fig. 16-27B,D); its density varies between individuals. In the submucosa, the ganglion cells have a tendency to concentrate in the vicinity of the muscularis mucosae forming the submucosal (Meissner’s) plexus and also immediately adjacent to the muscularis propria (deep submucosal or Henle’s plexus).56 The Henle’s plexus is more easily recognized in some animals species than humans. The ganglion cells are at least in part parasympathetic in origin, while both preganglionic and postganglionic parasympathetic and postganglionic sympathetic fibers are present in the plexi. A variety of hormonal neurotransmitters have been demonstrated, including not only acetylcholine and adrenergic fibers, but also substance P, vasoactive intestinal peptide, neuropeptide y, and calcitonin gene-related peptide. These specific transmitters do not correspond to the parasympathetic or sympathetic parts of the plexus.
Interstitial Cells of Cajal The term interstitial cell was applied by Cajal in 1893 to a cell that was believed to be a primitive neural cell with several long, anastomosing processes,57 and is probably the same cell earlier described by von Kollier in 1867.58 Most of the early work was carried out in nonhuman mammals, primarily rodents. Subsequently, similar cells have been described besides the GI tract in a variety of organs including salivary gland, gall baldder,
pancreas, and genitourinary tract.59, 60 These cells generate the slow wave of depolarization and represent the pacemaker cells of bowel peristalsis in the GI tract.61, 62 ICCs form a network throughout the gut from which electrical slow wave activity is propagated. If this network is broken, then two regions of muscle will function independently. Slow waves in the GI tract spread from ICCs to smooth muscle cells. The resulting depolarization initiates calcium ion entry into the cell and then contraction. Slow waves organize gut contractions into phasic contractions that result in peristalsis and segmentation. The frequency of ICC pacemaker activity differs in different regions of the GI tract, and is 3 per minute in the stomach, 12 per minute in the duodenum, 10 per minute in the ileum, and 3 per minute in the colon. ICCs also mediate neural input from enteric motor neurons and express mechanosensitive mechanisms that cause these cells to respond to stretch by affecting the resting potential of ICCs. Animals lacking ICC have greatly reduced responses to acetylcholine, released from excitatory motor neurons, and to the neurotransmitter nitric oxide, that is released from inhibitory motor neurons. Loss of ICC in disease may therefore interrupt normal neural control of GI contractions and lead to functional disorders that likely include irritable bowel syndrome.Their function is in turn therefore modulated by intrinsic and extrinsic neural inputs.
pancreas, and genitourinary tract.59, 60 These cells generate the slow wave of depolarization and represent the pacemaker cells of bowel peristalsis in the GI tract.61, 62 ICCs form a network throughout the gut from which electrical slow wave activity is propagated. If this network is broken, then two regions of muscle will function independently. Slow waves in the GI tract spread from ICCs to smooth muscle cells. The resulting depolarization initiates calcium ion entry into the cell and then contraction. Slow waves organize gut contractions into phasic contractions that result in peristalsis and segmentation. The frequency of ICC pacemaker activity differs in different regions of the GI tract, and is 3 per minute in the stomach, 12 per minute in the duodenum, 10 per minute in the ileum, and 3 per minute in the colon. ICCs also mediate neural input from enteric motor neurons and express mechanosensitive mechanisms that cause these cells to respond to stretch by affecting the resting potential of ICCs. Animals lacking ICC have greatly reduced responses to acetylcholine, released from excitatory motor neurons, and to the neurotransmitter nitric oxide, that is released from inhibitory motor neurons. Loss of ICC in disease may therefore interrupt normal neural control of GI contractions and lead to functional disorders that likely include irritable bowel syndrome.Their function is in turn therefore modulated by intrinsic and extrinsic neural inputs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree