Fig. 8.1
The rate of publication on the topic of single-port laparoscopic surgery has increased drastically in all surgical disciplines (red) and in colon and rectal surgery (blue) (Reprinted from [3])
In this chapter, we will describe the surgical technique but also data arising from the literature about the real benefits the technique is expected to bring to the patients.
8.1 Anesthesia for Laparoscopy
The physiological changes associated with the creation of a pneumoperitoneum and positioning of the patient during laparoscopy must be appreciated and compensated for, to avoid complications and adverse outcome, which may have an insidious onset.
Laparoscopic surgery has been traditionally contraindicated in patients with severe ischemic disease, valvular disease, and significant renal dysfunction or end-stage respiratory disease. Nowadays, absolute contraindications to the laparoscopic approach are limited to raised intracranial pressure, severe uncorrected hypovolemia, and acute heart failure. An accurate preoperative balance between the advantages of the laparoscopic approach and intraoperative risks is crucial to maximize the benefits of the technique [5].
8.1.1 Conduct of Anesthesia
The safer technique for airway management involves placement of a cuffed oral tracheal tube, neuromuscular relaxation, and positive-pressure ventilation. It is recommended that mask ventilation before intubation should be minimized to avoid gastric distension. The insertion of a nasogastric tube may be required not only to deflate the stomach but also to reduce the incidence of postoperative nausea and vomiting that can be very distressing for the patients [6]. The use of the laryngeal mask airway remains controversial. Locoregional anesthesia is limited to selected cases [7].
8.1.2 Ventilation
Traditional volume control modalities use constant flow to deliver a preset tidal volume and ensure an adequate minute volume at the expense of an increased risk of barotrauma and high inflation pressure. The use of pressure-controlled modalities minimizes peak pressure and has been shown to provide improved areolar recruitment and oxygenation [8]. The mean airway pressure (Paw), both the highest airway pressure (peak) and the airway pressure during the plateau phase, must always be less than 30 cm H2O in order to avoid ventilatory-induced lung injury (VILI). In fact, in case of rise in PCO2, ventilation strategy must be adapted to the patient, preferring an increase of the respiratory rate and limiting tidal volume and insufflation pressure. Translating the concept of lung-protective ventilatory strategy from the adult respiratory distress syndrome context, the application of “an open lung” strategy consisting of a recruiting maneuver followed by the subsequent application of positive end-expiratory pressure (PEEP) has been suggested to effectively reexpand pneumoperitoneum-induced atelectasis and improve oxygenation during laparoscopic surgery [9].
8.1.3 Analgesia
By the nature of minimally invasive surgery, the pain is often short, yet intense, requiring opioid analgesia at some stage perioperatively. The use of systemic drugs (FANS and paracetamol), regional technique such as subdural, epidural, and more recently transversus abdominis plane block (TAP block), together with wound infiltration with local anesthetic are increasingly used as opiate-sparing techniques [10].
8.1.4 Monitoring
Standard monitoring during laparoscopic surgery does not differ from open surgery and includes noninvasive monitoring of blood pressure (NIBP), ECG, control of the neuromuscular block (TOF), bispectral analysis (BIS) for assessing the depth of the hypnotic component of anesthesia, end-tidal CO2 (EtCO2), and SaO2, supported by information available on modern anesthetic machines such as peak and plateau airway pressures and dynamic flow-volume loops.
Most anesthetists advocate the use of invasive arterial monitoring, particularly in those patients with cardiovascular comorbidities, which allows continuous analysis of arterial blood gas (ABG). In selected cases, due to the poor value of the central venous pressure in the assessment of preload, other devices such as esophageal Doppler monitor (EDM), transesophageal echocardiography, the Vigileo, and the PiCCO systems can be used to monitor cardiac output (CO) of the patients. Those methods are recently replacing the insertion of a pulmonary artery catheter in an attempt to reduce iatrogenic complications and improve the ease of CO monitoring.
The PiCCO system (pulse contour cardiac output) is based on a transpulmonary thermodilution curve and allows continuous monitoring of the CO as well as monitoring of the global end-diastolic volume (GEDV) and of the extravascular lung water (EVLW).
The Vigileo system (Vigileo, Edwards LLC) analyzes the entire arterial waveform, and it does not necessarily require a central line. Furthermore, it does not require an initial calibration and allows continuous monitoring of the CO, the stroke volume (SV), the systemic vascular resistance (SVR), and the stroke volume variation (SVV) which represents an important index of preload and of patient’s response to fluids.
NICOM (noninvasive cardiac output monitoring) (Cheetah Medical, Tel Aviv) is based on bioreactance measurements through external sensors positioned on patient’s chest. Through this fully noninvasive method, continuous monitoring of CO, SV, SVV, and total peripheral resistance (TPR) is possible.
8.2 Surgical Technique
The procedure is performed with the patient under general anesthesia, with endotracheal intubation. At surgery, prophylactic intravenous antibiotics are administered. Pneumoboots are placed bilaterally in the operative room and activated before induction. The pneumoboots remain in place until the patients are ambulating. Deep vein thrombosis prophylaxis is started 6 h after the end of the operation, prolonged during the entire hospital stay, and discontinued at home at different time interval depending on the underlying disease (benign or malignant).
The patient lies in supine position, the table is 30° tilted leftward, while the surgeon and the assistant stand at the left side of the patient, the latter holding the camera at the head of the patient. The laparoscopic track is positioned in front of the surgeons, while the scrub nurse can stay between the patient’s legs or at the left side of the surgeon (Fig. 8.2).

Fig. 8.2
Operative room setup
Access to the peritoneal cavity is obtained through a skin incision of about 25–30 mm either right around the upper edge of the umbilicus or dividing longitudinally the umbilicus itself. In this case, the umbilicus is grasped at its base and everted. A 25-mm fascial incision is made, the peritoneum is entered, and the single-incision laparoscopic system (SILS) port (Covidien, Norwalk, CT, USA) or the TriPort system (Olympus Surgical and Industrial America Inc., Center Valley, PA) is placed into the peritoneal cavity. Those ports include an insufflation attachment and, respectively, three or four access ports, which can be used for 12-mm and 5-mm trocars. Pneumoperitoneum is then established. Standard non-articulated instruments are generally introduced through the single-access device: a 5-mm 30° scope, and two 5-mm working instruments, usually a Johanne forceps and an energized device. The use of 30° scope is strongly recommended to keep the camera out of line with the operating surgeon’s instruments. Also a 5-mm flexible-tip high-definition laparoscope (Olympus KeyMed, Southend, UK) or a bariatric length camera could provide better ergonomics to the surgeon and better facilitation of camera operation.
Thirty-degree 10-mm optic can be used as well, but this will further reduce the working room and it will have to be replaced by a 5-mm optic when utilizing the stapler.
During dissection, the grasper and the energized device change port to ensure the best angle.
Initial laparoscopic exploration is performed, and the patient is then placed in a 20° Trendelenburg position. The ileocolic pedicle is exposed retracting the ileocolic junction with the left hand (Fig. 8.3). To facilitate the visualization of the ileocolic pedicle, especially in obese patients, a stay suture introduced through the abdominal wall with a straight needle can be positioned to lift up the transverse colon (Fig. 8.4).
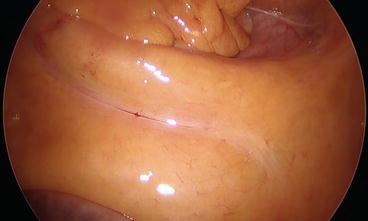
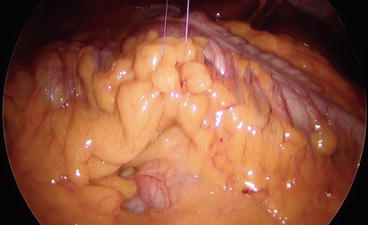

Fig. 8.3
The ileocolic pedicle is exposed by the traction exerted by the left hand on the ileocolic junction

Fig. 8.4
Transverse colon lifted up by a stay suture
Once the ileocolic pedicle is identified, it is lifted up to allow the opening of a window beneath the vessels, thus identifying the duodenum (Fig. 8.5). A medial-to-lateral dissection between Toldt and Gerota’s fascia is performed both with the use of an energy device and with the aid of a small swab. We have to remember, in fact, that this plane is a completely avascular plane and no vascular connection exists between the visceral mesentery and the retroperitoneal plane (Fig. 8.6). Thus, when dissecting this plane, one must be aware that the presence of a bleeding means that dissection is being conducted in the wrong plane. It may be useful to remember that when you go in the wrong plane, you are almost always going too posteriorly, putting retroperitoneal structures (e.g., ureter and gonadal vessels) at risk of injury.
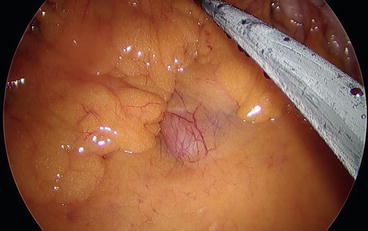
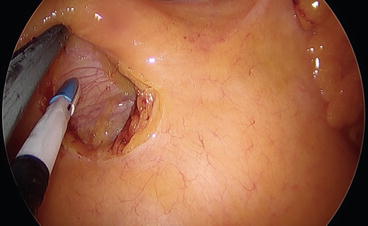

Fig. 8.5
The duodenum is visible in the field

Fig. 8.6
A window is opened beneath the ileocolic vessels and a medial-to-lateral dissection is performed between Toldt and Gerota’s fasciae
When the retroperitoneal plane is completely dissected free and the duodenum is well visualized in the field, the ileocolic pedicle can be sealed either by the LigaSure Advance (Covidien, Mansfield, MA, USA), the application of clips, or by the use of an Endo GIA loaded with a white cartridge (Figs. 8.7 and 8.8).
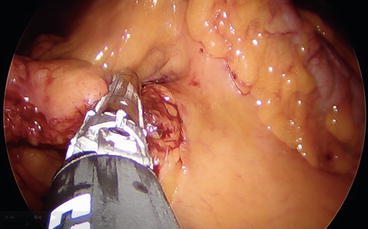
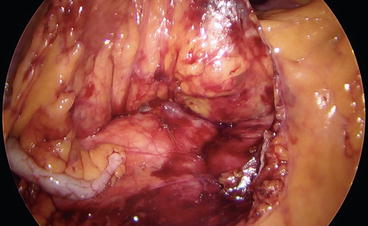

Fig. 8.7
Section of the ileocolic pedicle

Fig. 8.8
The staple line sealing the ileocolic vessels is well visible in the field with the duodenum and the Gerota’s fascia
The cecum is then mobilized, the lateral attachments are dissected free, and the mesentery is divided up to the terminal ileum, which is then sectioned with an Endo GIA (Figs. 8.9 and 8.10).
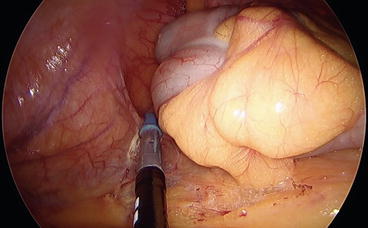
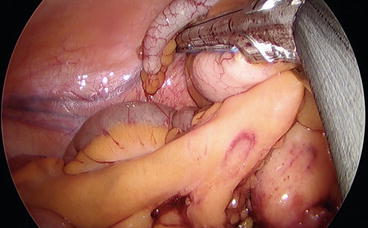

Fig. 8.9
The cecum and the ascending colon are mobilized by cutting the lateral attachments. Please note in this case that the dissecting instrument is in the left hand while the traction is performed with the right hand of the surgeon

Fig. 8.10
The terminal ileum is transected with an Endo GIA
To visualize the hepatic flexure, the grasper in the left hand can sweep the right colon medially and anteriorly which will display the hepatocolic attachments and the duodenum. Once the hepatic flexure is mobilized, the omentum attached to the specimen is divided. The transverse colon is sectioned using an endoscopic linear stapler, and the specimen can be positioned in an endobag to be removed through the same umbilical incision together with the access device. A side-to-side extracorporeal anastomosis is then performed up to surgeon’s preference. Alternatively, the specimen is positioned on the right lobe of the liver in an endobag, and a side-to-side ileocolic anastomosis is fashioned by mechanical stapling, closing the defect with interrupted stitches tied extracorporeally.
If previously removed, the access device is then reinserted to allow inspection of the peritoneal cavity and careful hemostasis. The fascial defect is then closed using interrupted absorbable sutures.
8.3 What We Know
Up to date, all data emerging from the literature support the idea that right colectomy with the single-access technique is not only feasible, but it is probably, together with total colectomy with end ileostomy, most clearly suited to single-port procedure, as it mandates an extraction site of equivalent size to a single-port apparatus. In fact in a recent article, Costedio and Remzi, the first who described a single-port right colectomy [13], stated that colorectal surgery is a natural place for single-access surgery and, as technology advances, it is likely that there will be a niche that benefits from single-port laparoscopy [14].
The theoretical benefits of SILC include improved cosmesis, decreased pain, and shorter length of stay.
Randomized trial and meta-analysis in single-access laparoscopic cholecystectomy failed to demonstrate a reduction in postoperative pain compared to conventional laparoscopic cholecystectomy [15–17], but data are lacking in the colorectal literature. Furthermore, in the authors’ opinion, it could be very difficult, even in properly conducted randomized trial, to demonstrate a significant reduction of pain when comparing right SILC with conventional 3-port laparoscopic right colectomy.
Despite the lack of data, all the published studies [18–34] suggest that SILC is feasible and safe and has short-term and oncologic outcome (in terms of free margins and number of nodes harvest) comparable to multiport laparoscopy.
We will briefly analyze the published data to discuss if and how those data support this statement.
We were able to analyze the results of 11 case-controlled series, published between 2010 and 2013 [18–28]. The results of those series are shown in Table 8.1. There were 361 SILS procedures compared with 510 conventional multiport colectomies (CMC). Rate of conversion from SILS to multiport laparoscopy was 5.8 % (21 cases), whereas conversion to open surgery occurred in only 1.3 % of the whole series (five patients). Rate of conversion to open surgery in the CMC was 4.5 % (23 patients).
Table 8.1
From 11 case-controlled series published between 2010 and 2013, 361 SILS procedures are compared with 510 CMC
Authors | SILC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
N° | Exclusion criteria | Conversion (port added/open surgery) | LOS | Operative time | Mortality | Morbidity | Age | BMI | Device | |
Rosati et al. [19] | 50 | Tumor >3,5 cm, contraindications to laparoscopic surgery | 0 | 6***(4–16) | 160 (115–210) | 0 | 8 %****(4/50) | 65 (36–88) | nr | Endocone |
Chew et al. [20] | 40 | Emergency | 3 | 5 (4–15) | 95 (45–180) | 0 | 23 % (9/40) | 63 (41–84) | 22.3 (16.1–34.9) | SSL (53 %) |
2 | SILS (2 %) | |||||||||
Yun et al. [18] | 66 | Benign disease | 0 | 8 ± 4 (5–27) | 155 ± 45******* (97–455) | Not cleara | 9.1 % (6/66) | 61 ± 11 (33–80) | 23.82 ± 2.81 (18.5–31.9) | Handmade glove port |
1 | ||||||||||
Egi et al. [25] | 10 | None
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||||||





