Background
Testicular cancer is the most common solid malignancy in males between the ages of 20 and 35 and primary germ cell tumors (GCT) are by far the most common histological type. Approximately 60% of GCT are pure seminoma, 30% are nonseminomatous GCT (NGCT), and 10% are mixed tumors [1,2]. In the United States, 8000 new cases and 390 deaths from the disease have been projected for 2008 [3]. Following orchidectomy, most seminoma patients (70–80%) present with no radiological evidence of disease (stage I), 15–20% have infradiaphragmatic lymph node involvement (stage II) and 5% present with distant metastatic disease (stage III). Therefore, in the United States in 2008, there will be approximately 4800 cases of seminoma, approximately 4100 of which will have stage I disease.
Management options for stage I seminoma include radiotherapy, surveillance, and adjuvant chemotherapy. For the past 65 years, the standard management of patients with stage I seminoma has been adjuvant retroperitoneal radiotherapy. Although providing excellent long-term results with virtually 100% local control in the abdomen and pelvis, this approach has been associated with increased risk of late gonadal toxicity, development of second malignancies and an increased risk of cardiovascular disease [4–9]. Various approaches have been investigated in efforts to reduce radiation-induced long-term morbidity, including reducing the radiation dose and treatment volume, as well as alternative management strategies including surveillance (including risk-adapted surveillance) and adjuvant chemotherapy [10–13].
It has been suggested that more patients with stage I seminoma will die of their treatment than of their cancer, so the thrust of modern management is to maintain the current 100% cure rate while minimizing the burden of treatment [9]. This chapter will discuss management of stage I seminoma, and outline and grade the evidence for our recommendations [14].
The Medline and EMBASE databases were searched for evidence using the following text, MeSH, and EMBASE subject headings: “testicular neoplasms,” “testicular cancer,” “neoplasms,” “germ cell and embryonal,” “seminoma,” “germinoma,” “dysgerminoma,” and “germ cell tumor.” These results were combined with the terms “radiotherapy,” “surveillance,” “watchful waiting,” “chemotherapy,” and “drug therapy” to provide a base pool of literature on the treatment of stage I seminoma, with the total results being limited to human studies published from 1981 through to September 2008.
Clinical question 36.1
Which patient, pathological and treatment factors are significant in selecting a management plan for a patient with stage I seminoma?
The evidence
A prognostic factor is a variable that can account for some of the heterogeneity associated with the expected course and outcome for a patient with a specific disease [15]. Thus, prognostic factors contribute to our best forecast of the behavior of cancer. This knowledge of outcome, or prognosis, forms an integral part of the decision-making process in medicine and formulating a management plan for any patient with stage I seminoma, or indeed any medical condition, requires a careful assessment of three principal groups of prognostic factors: host-related factors (factors inherent to the patient), tumor-related factors (the most common is pathological factors), and environment-related factors (principally treatment factors) [15].
In terms of selecting a management plan for a patient with stage I seminoma, two patient-related factors are of primary importance. First is the presence of co-existing illnesses that would largely preclude certain treatment options. Examples here would include inflammatory bowel disease or acquired immunodeficiency syndrome, which would rule out consideration of the use of adjuvant radiation therapy. In addition, the willingness or ability of the patient to be compliant to medical advice must be considered especially if surveillance with no adjuvant treatment is being considered as this approach entails multiple follow-up visits with repeated imaging over a 5–10-year time frame. Detection of disease recurrence at an early stage is important and while overall survival may not be affected because of the efficacy of salvage therapies, the type and therefore the morbidity of treatment are determined by the extent of disease at relapse.
Pathological prognostic factors for relapse have been studied in a number of surveillance series (Table 36.1). In the first series of surveillance patients, the Royal Marsden Hospital group reported on 103 patients and the only significant factor predicting relapse was the presence of lymphatic and/or vascular invasion (9% vs 17% relapse rate) [16]. On multivariate analysis in the Princess Margaret Hospital series of 201 patients, age and tumor size predicted for relapse, while small vessel invasion (SVI) approached statistical significance [17]. In a large Danish series of 261 patients, tumor size was the only significant predictive factor for disease recurrence [18]. A pooled analysis of four large surveillance series using individual patient data from four centers – Royal Marsden Hospital, Danish Testicular Cancer Study Group, Princess Margaret Hospital and the Royal London Hospital – has been performed [19]. Six hundred and thirty-eight patients were included in the study and on multivariate analysis, tumor size and rete testis invasion were identified as the only factors that predicted for relapse. An incremental rise in 5-year relapse rate was noted in the presence of zero, one or both of these risk factors (12.2%, 15.9% and 31.5% respectively; Figure 36.1). The hazard ratio (HR) for relapse with a tumor size > 4 cm was 2.0 (95% confidence interval (CI) 1.3–3.2) relative to baseline (tumor size < 4 cm and no rete testis invasion; see Table 36.1). The hazard ratio for rete testis involvement was 1.7 (95% CI 1.1–2.6) and with both adverse prognostic factors present, the hazard ratio for relapse was 3.4 (95% CI 2.0–6.1).
Table 36.1 Prognostic factors for relapse in surveillance series
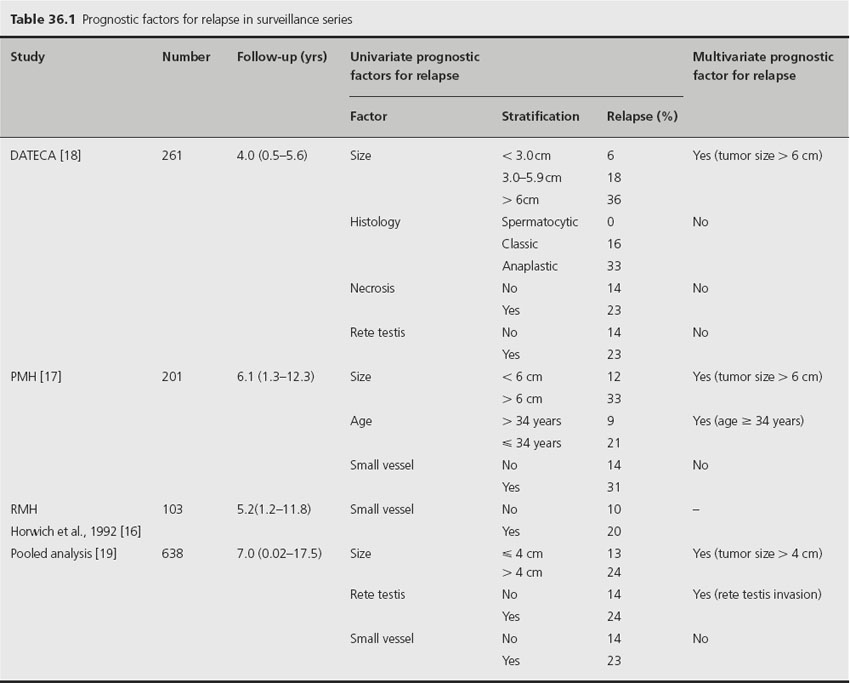
Figure 36.1 Relapse-free rate based on number of adverse prognostic factors. Reproduced from Warde et al. [19] with permission from the American Society of Clinical Oncology
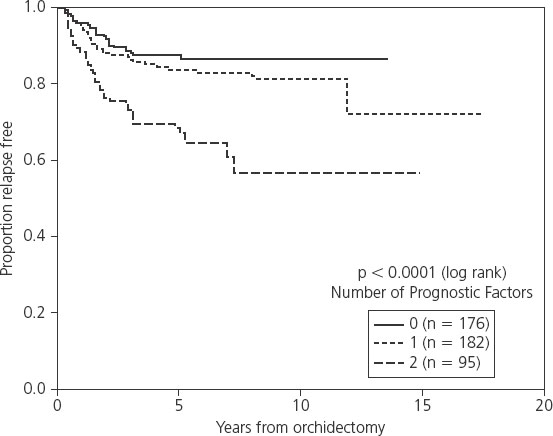
This prognostic model has not being validated in an independent dataset. However, a number of other reports give indirect support to the importance of tumor size and rete testis invasion as prognostic factors for relapse on surveillance. A recent retrospective review assessing factors predictive of metastasis at diagnosis indicated that tumor size > 6 cm and rete testis involvement predicted for the presence of more advanced disease at diagnosis [20]. In addition, a risk-adapted treatment study using tumor size > 4 cm and rete testis invasion as indicators for treatment was implemented in the second Spanish Germ Cell Co-operative Group (SGCCG) study [10]. Patients with no risk factors were managed with surveillance and 6% relapsed, similar to the expected percentage derived from the multi-institutional analysis prognostic model.
The most important treatment-related factor in selecting management for a patient with stage I seminoma is the late toxicity of adjuvant treatment, in particular radiation therapy. Other treatment-related factors include distance from the treatment center, which may influence the acceptability of surveillance as a management option.
Testicular germinal epithelium is extremely sensitive to ionizing radiation. Scatter dose to the contralateral testis may be significant and may cause severe depression of spermatogenesis and compromise future fertility. A radiation dose between 20 and 50 cGy may produce temporary aspermia, while higher doses may preclude recovery of spermatogenesis [21,22]. Although scrotal shielding should be used in every patient to reduce scattered radiation dose to the contralateral testis, it cannot ensure protection of spermatogenesis in all patients. A study of 451 consecutive testicular cancer patients in France demonstrated that the cumulative conception rates for patients who received radiotherapy were significantly lower compared with the rates for patients who received chemotherapy [23].
An increased risk of second nontesticular cancers after radiation therapy (RT) has been documented in a number of studies, and since this increased risk is expressed more than 10–15 years following RT, it is often not apparent in series with shorter follow-up [24]. Zagars et al. have reported an increased cancer-specific standardized mortality ratio (SMR) in 453 long-term survivors of radiation treatment for testicular seminoma [4]. A Dutch population-based study of 2707 testicular cancer survivors with a median follow-up of 17.6 years showed that the rate of second nontesticular cancers was 2.6-fold increased as compared to surgery alone [5]. A recent report combined 14 population-based registries including 10,534 patients with seminoma (all stages) treated with radiotherapy [7]. Compared with matched cohorts from corresponding registries, the overall relative risk of a second nontesticular malignancy was 2.0 (95% CI 1.8–2.2). For a 35-year-old patient with seminoma, the cumulative 40-year risk of a second malignancy was 36%, compared with 23% in the normal population.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








