Fig. 56.1
Endoscopic view of a complete clinical response
2.
A subtle loss of pliability of the rectal wall harboring the scar; usually observed during manual insufflations at proctoscopy with light stiffness of the wall. In the context of no additional positive findings of residual cancer, this may also be considered as a feature of cCR.
3.
Telangiectasia (small derogative blood vessels seen on the rectal mucosa at the area previously harboring the primary cancer) is also frequently observed in complete clinical responders, even in long-term follow-up.
4.
Whenever a tumor cannot be felt or seen, patients should be considered as complete clinical responders.
Alternatively, the following findings should be considered as incomplete clinical response and therefore warrant immediate surgical resection. Even though this may lead to a proportion of patients with pCR despite clinical findings of persistent cancer, it seems to be the safest procedure.
1.
Any residual deep ulceration with or without a necrotic center.
2.
Any superficial ulcer, irregularity, even in the presence of only mucosal ulceration.
3.
Any palpable nodule, defined by digital rectal examination, even in the presence of mucosal complete integrity.
In any of these situations, a surgical action is warranted, at least for diagnostic purposes. A non-surgical approach in this scenario is not recommended (Fig. 56.2).
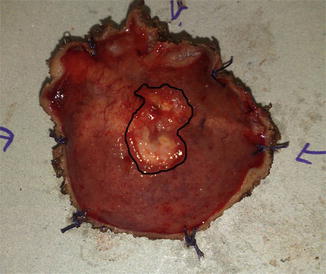

Fig. 56.2
Surgical specimen resected with TEM (Transanal Endoscopic Microsurgery) showing an incomplete response
One recent study has investigated the impact of the use of these stringent criteria in selecting patients likely to have a complete pathological response. In this study, simply by looking at specimen photographs, surgeons were asked to indicate complete pathological responders. Curiously, these criteria led to positive and negative predictive values ≥90 % for the identification of complete responders without any tactile information obtained from DRE, biopsies or radiological imaging [18].
56.4 Imaging Studies
Radiological assessment of response is of paramount importance for the appropriate selection of patients for an alternative treatment strategy such as the “Watch & Wait” approach following a complete clinical response. Not only they provide assessment of the primary tumor (within the rectal wall) but also of the mesorectal lymph nodes that are not accessible to clinical examination alone and a possible site for metastatic disease even in the presence of complete primary tumor regression (ypT0).
Basically, the same imaging modalities used for initial staging can be used for tumor response assessment. Endorectal Ultrasound (ERUS), has been studied for this purpose and in a recent report with 60 patients, the overall accuracy of the method for T staging was less than 40 % when assessment was performed after 45 days from CRT completion [19]. Assessment at 6–8 weeks may have improved accuracy to 75 %. However, identification of ypT0 was particularly poor [20].
Magnetic resonance imaging (MRI) is now considered standard for primary tumor staging and assessment of response after CRT. Findings of areas with low signal intensity replacing the area of the previous tumor or even no detectable abnormalities in MRI are consistent with radiological features of a complete clinical response. Three different patterns of low-signal intensity have been described in patients with complete clinical response: minimal fibrosis, transmural fibrosis and irregular fibrosis [21]. Also, histological tumor regression is currently estimated by MRI imaging with a classification inspired by tumor regression grading systems. This MRI “tumor regression grade” classification proved to correlate well with survival [22].
Diffusion-weighted MRI (DWI) is a functional MR imaging technique that uses differences in the extracellular movement of water protons to discriminate between tissues of varying cellularity. In tissues with increased cellularity (neoplasia), the diffusion of water is restricted, resulting in remaining high signal intensity on DWI-MR. In recent studies, the diagnostic performance for predicting a pathologic complete tumor response was improved with DWI MRI compared with standard MRI in nearly 20 %, reaching ≥90 % overall accuracy [23].
Positron-emission tomography (PET) with computed tomography (CT) may provide additional information regarding metabolic activity in tumors after neoadjuvant CRT. In addition to the visual identification of FDG uptake within the area of the rectal wall harboring the tumor or within the mesorectum, PET/CT allows the estimation of the metabolism profile. Standard uptake values (SUV) are direct estimations of tissue metabolism and may be used for the distinction of residual inflammatory changes and residual cancer. Measurement of SUV in two different intervals from FDG injection is routinely performed (1 and 3 h) and allows two distinct patterns (dual time) of metabolism. Increase in SUV (between 1 and 3 h) suggests the presence of residual cancer whereas decrease suggests inflammatory or fibrotic changes [24].
Several studies have suggested the use of PET/CT in assessing tumor response to CRT with conflicting results.[25–28]. In our experience, PET/CT when performed 12 weeks after chemoradiation completion was able to predict Complete Response with an accuracy of 85 % [29] (Fig. 56.3).
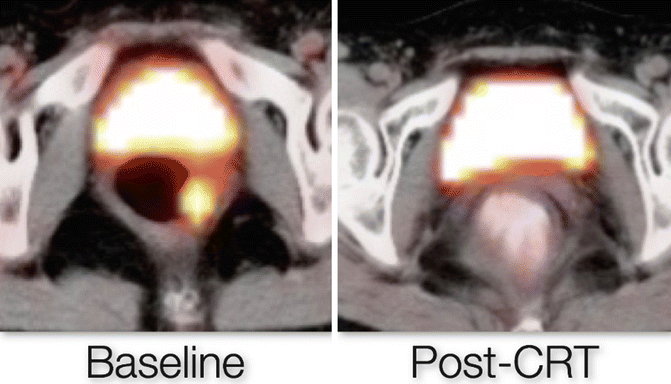

Fig. 56.3
PET/CT of a complete clinical response patient before and after CRT
56.5 CEA
The determination of CEA levels before and after CRT may be useful during assessment of tumor response. In one retrospective report of patients undergoing different CRT regimens it was showed that a pre treatment CEA level <2.5 ng/dl was predictor of ypCR [30].
An increase in CEA levels or persistence of at least 70 % from baseline has been suggested as predictor of worse outcome in patients with CEA levels >6 ng/ml at baseline [31]. Also, different cutoff values have been considered for patients undergoing CRT when compared to standard colorectal cancer patients. A retrospective analysis of 109 patients undergoing neoadjuvant therapy, identified a cutoff value for CEA <2.7 ng/ml at 4 weeks from RT completion to be predictor of tumor regression [32].
In our experience, no correlation with both pre-treatment CEA and variation between pre and post treatment CEA levels and tumor response or oncological outcomes was detected. On the other hand, a post-CRT level <5 ng/ml after at least 8 weeks from CRT completion was associated with increased rates of earlier disease stage and complete tumor regression [33].
56.6 Endoscopic Biopsies After CRT
During endoscopic evaluation of a residual lesion, forcep’s biopsies are frequently performed and considered by many to be useful in assessment of tumor response. Even though a positive result implies obvious persistence of residual tumor, negative results may warrant cautious interpretation.
In a retrospective review of patients undergoing neoadjuvant CRT and significant tumor downsizing, post-CRT biopsies resulted in a negative predictive value of 21 %. In other words, a negative biopsy truly identified a pCR in only 21 % of cases [34].
Nevertheless, endoscopic biopsies may have a role in the management of these patients since a positive biopsy is sometimes a useful argument to convince patients who refuse radical surgery to accept treatment in the presence of residual cancer despite significant symptom relief.
56.7 Factors Associated with Tumor Response After CRT
Tumor response to CRT is not uniform and many factors may play a role. The specific CRT regimen and interval between CRT completion and assessment of response seem to be as important as tumor and patient-related characteristics.
56.8 Chemoradiation Regimen
Fractionated long course chemoradiation followed by surgery after 6–8 weeks or pelvic short-course irradiation with 25 Gy in five fractions followed by immediate surgery are the two most used regimens in the preoperative treatment of patients with resectable T3–4 rectal cancer. Benefits in local disease control seem to be equivalent between them, but there are significant differences in terms of tumor downstaging [35].
The rates of pCR are significantly lower in patients undergoing short-course RT, when compared with those undergoing long-course. At first glance, the long-course regimen includes chemotherapy and this could be determinant for that difference. It should also be considered that damaged cancer cells need time to undergo necrosis after radiotherapy and usually patients undergoing short-course RT, surgery is performed 1 week after RT completion whereas long-course CRT is followed by radical surgery after at least 6–8 weeks. In fact, one randomized study has demonstrated that pathological regression is more pronounced in the presence of combination of chemo and radiation therapy when compared to radiation therapy alone [36]. A very recent systematic revision from Cochrane, including five randomized controlled trials comparing CRT vs RT alone for resectable stage II or III rectal cancer, showed that the first approach was associated with a higher rates of ypCR [37].
A review of phase II and III studies using different neoadjuvant CRT regimens for rectal cancer including >4,000 patients in 71 studies with different regimens, reported a ypT0 rate that varied from 0 to 42 %. In this review, pCR was significantly associated with the delivery of radiation doses higher than 45-Gy, 5-FU regimens with continuous infusion and the use of a second drug (being oxaliplatin the most frequent additional drug) [38].
The association of higher rates of pCR and the addition oxaliplatin to the traditional scheme of 5-FU has been strongly questioned in light of the results of a recent prospective randomized trial that showed that this addition was not associated with better rates of pCR. Instead, patients treated with oxaliplatin experienced significantly more treatment-related toxicities [39].
Targeted biological drugs used for metastatic disease, such as bevazicumab and cetuximab, were included in phase I and II studies in combination with other drugs aiming to increase response rates. Results, however, were disappointing. A review of these trials also suggested a sub-additive interaction between capecitabine, oxaliplatin, and cetuximab as reflected by decreased rate of pCR (9 vs. 16 %) and significant decrease in tumor regression grades (more than 50 % of tumor regression) among surgical specimens from these patients when compared with patients undergoing treatment with capecitabine and oxaliplatin alone CRT regimens [40]. It is not clear whether the inclusion of patients according to the K-ras status could have any influence in response to neoadjuvant CRT with this triple approach [41].
56.9 Timing for Tumor Response Assessment
The optimal interval between CRT and surgery has not yet been identified for rectal cancer. The Lyon R90–01 study is so far the only randomized trial that evaluated the time interval between the completion of CRT therapy and surgery (2 weeks vs 6 weeks), and demonstrated improved T and N downshift with the longer interval (6 weeks) [42]. In addition, retrospective studies echo the finding that a longer interval to surgery may actually increase pCR rates [43]. In a recent review of the Cleveland Clinic experience, there was a steep increase in the pCR rate after 7 weeks from CRT completion; this increase reached a plateau only after 12 weeks [7].
A longer interval to surgery may confer another benefit. A review of patients treated with different intervals after neoadjuvant therapy suggested that delayed surgical resection was associated with decreased perioperative morbidity and no oncologic compromise [44]. In fact, preliminary results from a prospective non-randomized trial showed that in presence of tumor response to CRT (5FU + RT) the addition of two cycles of FOLFOX-6 scheme and delaying surgery for 12 weeks resulted in a modest increase of ypT0 rate without increasing postoperative complications [45]. Altogether, these results may suggest that 12-week intervals are perhaps ideal prior to assessment of tumor response following CRT completion. There are currently ongoing randomized trials addressing the question of 6 or 12-week intervals following CRT completion in rectal cancer that are likely to lead to more definitive conclusions in the near future [46].
Still, there is a possibility that not all patients would benefit from waiting more than 6 weeks, as suggested by a recent study using PET/CT to evaluate tumor metabolism [47]. Some patients may actually develop increase in tumor metabolism between 6 and 12 weeks suggesting no actual benefit. However, it is not clear whether this increment in metabolism is detrimental and that interruption (surgery) at 6 weeks provides any benefit for these patients.
56.10 Tumor Features and Biology
Several aspects of the primary rectal cancer such as tumor height, extension and initial disease staging, have been considered to be predictors of tumor response or complete pathological response to neoadjuvant treatment. Even though very few studies have included patients with cT2N0 rectal cancer treated by neoadjuvant CRT, these tumors seem to be more likely to develop complete clinical response [29]. The ACOSOG trial that included cT2N0 for neoadjuvant CRT followed by local excision resulted in a surprisingly high pCR (ypT0) rate of 44 % [48].
In one retrospective study of over 500 patients tumor extension was an independent predictive factor of pCR after neoadjuvant CRT. In this study, circumferential tumor extent of <60 % was a significant predictor of pCR. Even though tumor distance from the anal verge was not a predictor of pCR, tumors located in the distal 5 cm of the rectum were more likely to develop greater tumor downstaging [49]. More recently, another study identified that high pre-treatment CEA levels and tumors located in the distal 5 cm of the rectum were less likely to develop pCR [50].
In the near future, molecular biology will help the identification of tumors that will respond completely to CRT. Currently available studies in this regard however have failed to demonstrate a useful gene signature capable of predicting response to CRT and significant limitations have been identified. First, studies have used different endpoints of response (complete response in some and “good” response in others). Second, different platforms for gene expression are currently available and were actually used. Finally, there was no overlap in terms of genes predicting response in each of these studies [51–54].
56.11 The Watch-and-Wait Protocol
Patients with complete tumor regression, either after clinical assessment (cCR) or after transanal local excision (ypT0), have been enrolled in a strict follow-up program with no immediate surgery (Fig. 56.4). It is critical the adherence to the program because distinguishing between complete and near-complete responses may be difficult in some situations and final decision is only possible after a few follow-up visits. This is why an empirical 12-month probation period has been suggested where only patients that sustain a complete clinical response are considered as true cCR’s [55].
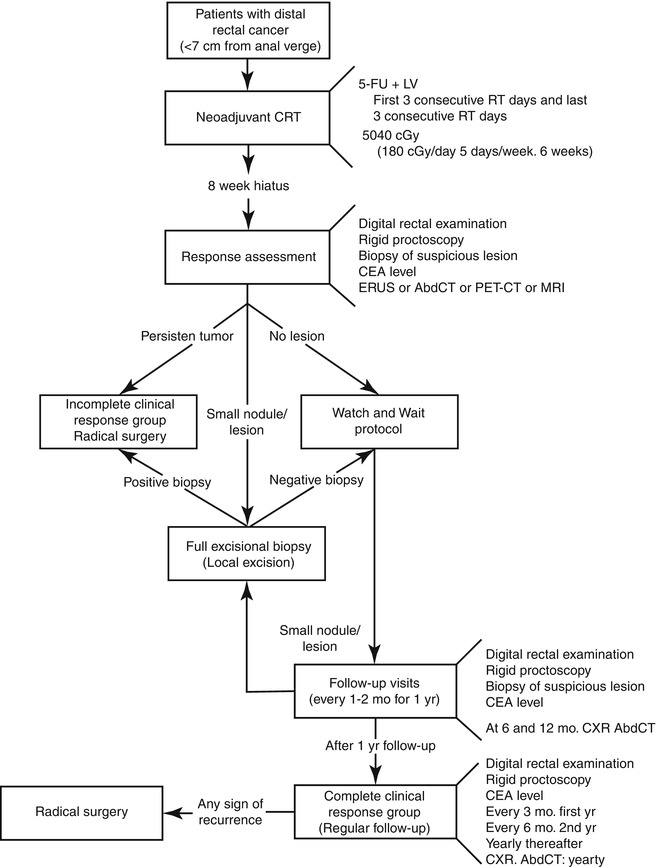

Fig. 56.4
The watch-and-wait algorithm
The algorithm includes monthly follow-up visits with digital rectal examination and rigid proctoscopy in every visit for the first 3 months and every 2–3 months during the rest of the first year. CEA levels are determined every 2 months. Radiological studies, including MRI or PET/CT are performed at the time of initial tumor response assessment and every 6 months if there are no signs of tumor recurrence. Patients are aware that complete clinical regression of their primary tumor may be temporary and tumor regrowth may occur at any time during follow-up. Small nodules or scars may develop over time and can be managed by full-thickness transanal excision (either by standard or Transanal Endoscopic Microsurgery techniques), primarily as a diagnostic approach. Patients with complete primary tumor response after FTLE (ypT0) are also considered as cCR and are not recommended to further resection.
In the case of obvious recurrence, radical surgery is strongly recommended.
After 1 year of sustained, complete clinical response, patients are recommended for follow-up visits every 3 months.
This treatment strategy has evolved since the beginning of our experience in 1991. Our accuracy in clinical assessment of tumor response has probably improved significantly with growing experience. At the beginning, patients were more frequently followed without immediate surgery when a near-complete clinical response was considered and expecting that time would lead to a complete clinical response. More recently, these patients have been more frequently assessed using full-thickness local excision (FTLE) as a diagnostic procedure, and according to the pathologic report managed by strict observation or referred to immediate radical surgery.
56.12 Results
In order to understand if there was any oncological benefit of radical surgery in the setting of complete tumor regression, a retrospective study was carried out at our Institution where patients with complete pathological response (pCR) managed by radical surgery were compared to patients with cCR managed non-operatively [56]. Patients managed by observation alone had similar outcomes to those managed by radical surgery in terms of long-term survival. Local recurrences were higher in the observation group. However, all recurrences were confined to the rectal wall and amenable to surgical salvage [57]. No exclusive pelvic relapses without endorectal component was observed.
A very similar study has recently been reported from another Institution revealing identical oncological outcomes with no survival benefit among patients with pCR managed by radical TME over patients with cCR managed by observation alone. Curiously, this study suggested a worse functional outcome among patients in the resection group (pCR) when compared to the “watch and wait” group [58].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








