Secondary Tumors of the Bladder
The bladder may be involved secondarily by tumors from adjacent sites such as the prostate, seminal vesicles, lower intestinal tract, and the female genital tract (1, 2, 3, 4). These tumors more commonly involve the bladder by direct extension and can also metastasize to the bladder. Malignant lymphoma or leukemia may also secondarily involve the bladder as part of a more disseminated or systemic manifestation (see Chapter 12). Less commonly, the bladder may be involved by metastatic tumor from distant sites. The diagnostic problems posed for the pathologist in cases of secondary involvement vary according to the morphology of the primary neoplasm.
TUMORS INVOLVING THE BLADDER PRIMARILY BY DIRECT EXTENSION
Carcinomas of the prostate, rectosigmoid, and uterine cervix account for the most common primary neoplasms involving the bladder by direct extension, although other cancers such as those from the endometrium, ovary, appendix, and other segments of the colon may rarely directly involve the bladder (3, 4, 8, 9).
PROSTATE
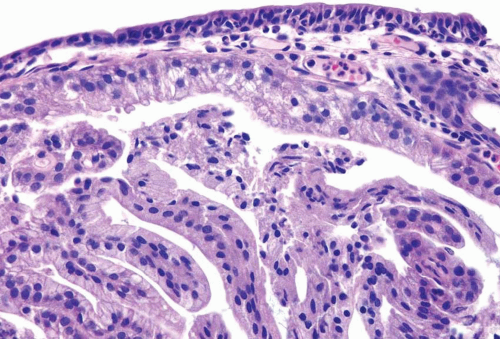
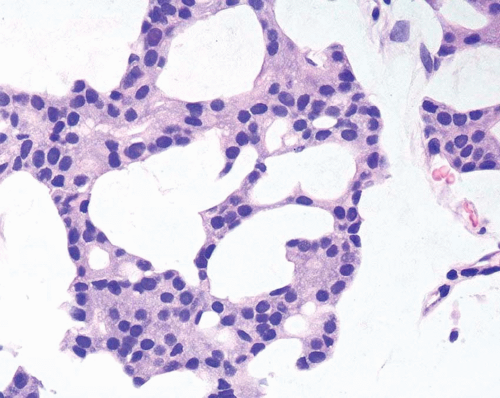
A common diagnostic problem is in differentiating within transurethral resection of the prostate (TURP) between a poorly differentiated urothelial carcinoma of the bladder and a poorly differentiated prostatic adenocarcinoma (5) (Figs. 13.1, 13.2 and 13.3) (efigs 13.1-13.8). The therapy of these two diseases differs significantly, making the distinction between these two entities crucial. Even in poorly differentiated prostatic carcinomas, there is typically relatively little pleomorphism or mitotic activity compared to poorly differentiated urothelial carcinoma. Poorly differentiated prostate cancers may have enlarged nuclei and prominent nucleoli, yet usually there is little variability in nuclear shape or size from one nucleus to another (Fig. 13.4). High-grade urothelial carcinomas often reveal marked pleomorphism, occasionally with tumor giant cells. A subtler finding is that the cytoplasm of prostatic adenocarcinoma is often very foamy and pale imparting a “soft” appearance. In contrast, urothelial carcinomas may demonstrate hard, glassy eosinophilic cytoplasm or more prominent squamous differentiation. The findings of infiltrating cords of cells or focal cribriform glandular differentiation are other features more typical of prostatic adenocarcinoma than urothelial carcinoma. Urothelial cancer tends to grow in nests, even when poorly differentiated. Although the above distinction between urothelial carcinoma and prostatic adenocarcinoma on H&E stained sections is valid for almost all cases, rare cases of prostate adenocarcinoma have marked pleomorphism identical to urothelial carcinoma (6). Consequently, in a poorly differentiated tumor involving the bladder and prostate without any glandular differentiation typical of prostate adenocarcinoma, the case should be worked up immunohistochemically.
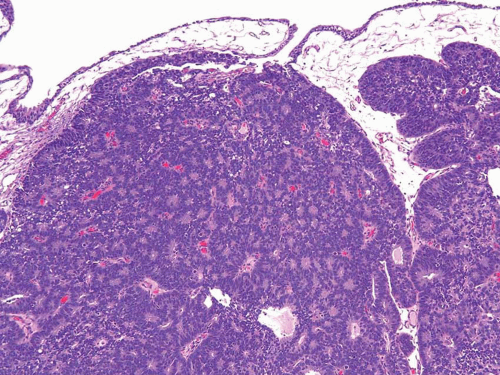 FIGURE 13.1 Metastatic prostate adenocarcinoma with poor cribriform glandular formation resembling rosettes undermining urothelium. |
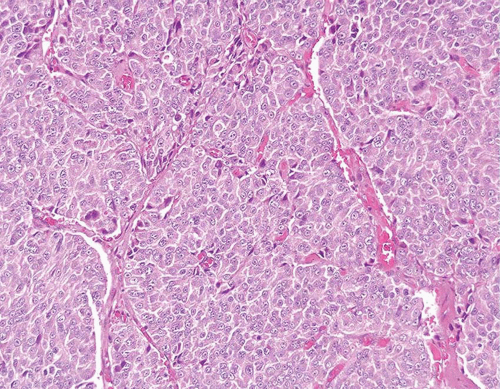 FIGURE 13.4 Poorly differentiated adenocarcinoma of the prostate lacking pleomorphism typically seen with urothelial carcinoma. |
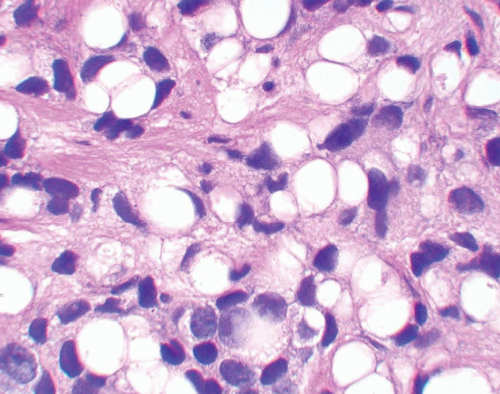
In contrast to bladder adenocarcinomas, adenocarcinoma of the prostate rarely contains mucin-positive signet cells. Some carcinomas of the prostate will have a signet-ring cell appearance, yet the vacuoles appear empty and do not contain intracytoplasmic mucin (Fig. 13.5) (7). Only a few cases of prostate cancer have been reported with mucin-positive signet cells (8, 9).
Almost 50% of the cystoprostatectomy specimens examinedfor urothelial carcinoma also contain adenocarcinoma of the prostate (10). Therefore, the finding in a TURP of a small focus of a well-differentiated adenocarcinoma of the prostate should not necessarily influence whether a separate focus of poorly differentiated tumor is urothelial carcinoma or adenocarcinoma of the prostate.
Approximately 90% of poorly differentiated prostatic adenocarcinomas show PSA and PSAP staining although it may be focal (11, 12, 13). While some studies claim superiority of PSA over PSAP in staining prostatic carcinoma, other articles have demonstrated poorly differentiated prostatic carcinomas that lacked PSA staining but still maintained their
immunoreactivity with antibodies to PSAP (12, 13, 14, 15). Monoclonal antibodies to PSAP have lower sensitivities than their polyclonal counterparts (16). In some cases of poorly differentiated prostatic adenocarcinomas with “poor” PSA staining, prostate-specific membrane antigen (PSMA) and p501S (Prostein) may be positive (17). However, a small percent of prostate cancers are negative for all markers. Therefore, the lack of immunoreactivity to prostate-specific markers in a poorly differentiated tumor within the prostate, especially if present in limited amount, does not exclude the diagnosis of a poorly differentiated prostatic adenocarcinoma (Table 13.1).
immunoreactivity with antibodies to PSAP (12, 13, 14, 15). Monoclonal antibodies to PSAP have lower sensitivities than their polyclonal counterparts (16). In some cases of poorly differentiated prostatic adenocarcinomas with “poor” PSA staining, prostate-specific membrane antigen (PSMA) and p501S (Prostein) may be positive (17). However, a small percent of prostate cancers are negative for all markers. Therefore, the lack of immunoreactivity to prostate-specific markers in a poorly differentiated tumor within the prostate, especially if present in limited amount, does not exclude the diagnosis of a poorly differentiated prostatic adenocarcinoma (Table 13.1).
With only a few exceptions, immunoperoxidase staining for “prostatic markers” is very specific for prostatic tissue. Adenocarcinomas of the bladder, whether as a pure tumor or with mixed urothelial carcinoma, do not express PSA and PSAP, when studied with monoclonal antibodies (18). A minority of bladder adenocarcinomas label with the prostate antigen P501S, yet not with the characteristic granular perinuclear staining. Rare cases of bladder adenocarcinoma show that PSMA membranous staining is indistinguishable from that seen in prostate cancer. In a poorly differentiated tumor occurring in the bladder and the prostate where the differential diagnosis is between high-grade prostatic adenocarcinoma and urothelial carcinoma, focal strong staining for PSA, PSAP, P501S, or PSMA can be used reliably to diagnose prostatic adenocarcinoma, since false positive staining has not been described in urothelial carcinomas (16, 19, 20).
In general, various cytokeratins (CK7, CK20, high molecular weight cytokeratin) show strong positivity in cases of urothelial carcinoma involving the prostate (Table 13.1). Although CK7 and CK20 are more frequently seen in urothelial carcinoma as compared to adenocarcinoma of the prostate, they may also be positive in adenocarcinoma of the prostate, such
that in our experience they are not that helpful in this differential diagnosis (10, 21, 22, 23, 24, 25, 26, 27, 28, 29). High molecular weight cytokeratin is positive in more than 90% of urothelial carcinomas (17, 30, 31). In contrast, high molecular weight cytokeratin is only rarely (8%) expressed, and usually in a very small percentage of cells, in adenocarcinoma of the prostate (17, 32). p63 is another useful marker in differentiating high-grade urothelial carcinoma from prostatic adenocarcinoma, with a greater specificity, albeit lower sensitivity for urothelial carcinoma compared to high molecular weight cytokeratin (100% specificity and 83% sensitivity) (17). Other markers that also appear highly specific but only of modest sensitivity for urothelial carcinoma include uroplakin and thrombomodulin (49%-69% sensitivity). We propose that a first line panel for this differential should include PSA (expected to be positive for prostate cancer), p63, and high-molecular-weight cytokeratin (expected to be negative in prostate cancer). In cases not fully resolved by this panel, a second line panel including PSMA and P501S (expected to be positive in prostate) and thrombomodulin (expected to be positive in bladder) should help resolve most cases.
that in our experience they are not that helpful in this differential diagnosis (10, 21, 22, 23, 24, 25, 26, 27, 28, 29). High molecular weight cytokeratin is positive in more than 90% of urothelial carcinomas (17, 30, 31). In contrast, high molecular weight cytokeratin is only rarely (8%) expressed, and usually in a very small percentage of cells, in adenocarcinoma of the prostate (17, 32). p63 is another useful marker in differentiating high-grade urothelial carcinoma from prostatic adenocarcinoma, with a greater specificity, albeit lower sensitivity for urothelial carcinoma compared to high molecular weight cytokeratin (100% specificity and 83% sensitivity) (17). Other markers that also appear highly specific but only of modest sensitivity for urothelial carcinoma include uroplakin and thrombomodulin (49%-69% sensitivity). We propose that a first line panel for this differential should include PSA (expected to be positive for prostate cancer), p63, and high-molecular-weight cytokeratin (expected to be negative in prostate cancer). In cases not fully resolved by this panel, a second line panel including PSMA and P501S (expected to be positive in prostate) and thrombomodulin (expected to be positive in bladder) should help resolve most cases.
TABLE 13.1 Immunohistochemistry Prostate and Bladder Carcinomas | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|