There is substantial interest in identifying patients with premalignant conditions such as Barrett’s esophagus (BE), to improve outcomes of subjects with esophageal adenocarcinoma. However, there is limited consensus on the rationale for screening, the appropriate target population, and optimal screening modality. Recent progress in the development and validation of minimally invasive tools for BE screening has reinvigorated interest in BE screening. BE risk scores combining clinical, anthropometric, and laboratory variables are being developed that may allow more precise targeting of screening to high-risk individuals. This article reviews and summarizes data on recent progress and challenges in screening for BE.
Key points
- •
Although Barrett’s esophagus (BE) is the precursor of most esophageal adenocarcinomas, most clinically diagnosed tumors are detected outside of surveillance programs.
- •
Early stage adenocarcinomas diagnosed in surveillance programs have better outcomes than those diagnosed after the onset of symptoms.
- •
Epidemiologic studies continue to indicate that most prevalent BE cases in the community remain undetected despite the increasing clinical use of endoscopy.
- •
Novel minimally invasive tools, such as the cytosponge and unsedated transnasal esophagoscopy, are promising tools for BE screening in the community.
- •
Need for the development of risk stratification tools for patients with BE is acute, and such tools are necessary for a BE screening program to be successful.
Introduction
Barrett’s esophagus (BE) is a recognized premalignant condition of the esophagus that can progress to esophageal adenocarcinoma (EAC). Despite advances in therapy, the survival of EAC diagnosed after onset of symptoms remains poor, with less than 20% survival at 5 years. The incidence of EAC continues to increase in Western countries, with an estimated 6-fold increase since 1975. Given that BE is the only known precursor of EAC, and a strong risk factor, screening for BE followed by endoscopic surveillance and treatment of dysplasia (or early neoplasia) has been thought to be an approach that can potentially reduce the incidence of EAC and improve survival. This article reviews and summarizes data on recent progress in screening of BE given the debate in the literature. It outlines the rationale for screening, recent advances in defining the target population, and technologies available for screening, and reviews recent advances in the risk stratification of progression in BE.
Introduction
Barrett’s esophagus (BE) is a recognized premalignant condition of the esophagus that can progress to esophageal adenocarcinoma (EAC). Despite advances in therapy, the survival of EAC diagnosed after onset of symptoms remains poor, with less than 20% survival at 5 years. The incidence of EAC continues to increase in Western countries, with an estimated 6-fold increase since 1975. Given that BE is the only known precursor of EAC, and a strong risk factor, screening for BE followed by endoscopic surveillance and treatment of dysplasia (or early neoplasia) has been thought to be an approach that can potentially reduce the incidence of EAC and improve survival. This article reviews and summarizes data on recent progress in screening of BE given the debate in the literature. It outlines the rationale for screening, recent advances in defining the target population, and technologies available for screening, and reviews recent advances in the risk stratification of progression in BE.
Rationale for screening
The primary purpose of a screening program is to detect premalignant or early stage neoplastic lesions, thereby providing an opportunity to improve outcomes and reduce mortality with early intervention. For BE, screening programs have been recommended by various gastrointestinal (GI) societies. However, these guidelines have been qualified as weak recommendations because of the lack of robust evidence. The reasons for discrepancy include lack of randomized, high-quality data showing the lack of reduction in EAC mortality with the implementation of screening and inefficient use of resources. The current reference standard technique for screening is the sedated upper endoscopy (sedated esophagogastroduodenoscopy [sEGD]), which is associated with significant costs to the system (eg, use of sedatives, postsedation recovery time, nursing) and to the patient (eg, loss of income for patients and caregivers who take time away from work to complete the procedure). Efforts have been made to find alternative options to sEGD, and these are discussed later.
The second aspect of screening consists of surveillance after the initial identification of BE, so that dysplastic lesions are identified and managed at an earlier stage. There are various grades of dysplasia in BE, with the lowest potential of malignancy being present within nondysplastic BE (NDBE). Although evidence in the past 5 years shows that the incidence of EAC in patients with NDBE is likely to be lower than was previously estimated, these numbers often exclude prevalent EAC detected at the time of initial endoscopy. A meta-analysis of 57 studies showed that the annual incidence of EAC in NDBE was 1 in 300 (0.33%). Approximately 7% of patients with BE on initial endoscopy had EAC. By eliminating prevalent EAC from the analysis, the data presented are skewed toward the lower incidence of BE-induced EAC and likely underestimate the true effect of screening on the population. The second concern is that the evidence is retrospective in nature, and associated with lead time bias (time from diagnosis of disease to presentation with symptoms), length time bias (increased survival time related to screening that identifies cases before onset of symptoms), and selection bias (patients willing to receive medical attention). It is uncertain what the true effect of screening is on EAC mortality, and whether it is a cost-effective program. However, a screening or surveillance program in principle can find early stages of disease, identify curable cancers, and still not have a mortality benefit. This possibility is well recognized from large screening studies of lung and ovarian cancers. Despite the lack of effect on cancer-specific mortality or all-cause mortality, such results have not reduced the use of transvaginal ultrasounds, cancer antigen 125, or chest radiography.
Screening provides the opportunity to identify tumors at an earlier stage compared with patients without screening. Dysplastic BE is a precursor lesion that, if found early and endoscopically treated, can eliminate the need for an esophagectomy. Five-year survival rates of early asymptomatic cancers (T1a and T1b) treated endoscopically are better than those of symptomatic cancers, in part because of the availability of endoscopic therapies such as endoscopic mucosal resection and radiofrequency ablation, which are the new definitive therapies for early EAC and dysplastic BE. This development has been positive, because esophagectomy continues to have high morbidity and mortality. The ability to prevent progression of dysplastic/early EAC is a potential incentive to identify patients at risk.
Because of the increasing incidence of EAC, in conjunction with high morbidity/mortality, interest in the population for screening is substantial. Approximately two-thirds of patients, when approached with information on BE and offered the option to be screened, were interested in screening in a population-based survey study. This finding may reflect the raised public profile that BE and EAC have obtained in the last decade. There are also various patient factors to consider following a diagnosis of BE. It has been associated with increased insurance premiums despite patients with BE dying primarily of nonesophageal causes, such as cardiovascular or pulmonary conditions. Life expectancy in patients with BE is comparable with that of individuals without BE. Moreover, patients with BE have reported reduced quality of life, and increased psychosocial stressors and use of health care resources (compared with patients with gastroesophageal reflux disease [GERD] and the general population). Cost analyses have suggested that 1-time screening endoscopy in patients with GERD is cost-effective if surveillance is limited to those who have dysplastic BE. All these factors have increased interest in appropriate screening strategies that are targeted to the correct population, without adding unnecessary anxiety to low-risk patients.
Target population
The high-risk group has the largest potential yield and benefit of screening. However, the target population for BE screening has not been well delineated. Population-based and autopsy studies have shown that only one-third of the prevalent BE cases in the general population are currently identified by clinically indicated endoscopy, despite the sharp increase in the volume of endoscopy in the United States. More than 90% of EAC cases are diagnosed outside of BE surveillance programs, despite the presence of BE histologically.
GERD is the strongest risk factor for BE and EAC. About 10% to 15% of patients with GERD are found to have BE on endoscopy. As obesity increases within North America, the prevalence of GERD has also increased. An update of a 2005 review reflecting on the prevalence of GERD symptoms showed a global increase in weekly symptom scores (10% increase in North America, 15% in Europe, 24% in the Middle East, and 5.5% increase in east Asia). The largest increase in GERD was in North America and east Asia. However, not all patients with BE endorse symptoms of GERD, with some studies reporting that 46% of subjects with BE were asymptomatic for reflux symptoms. The prevalence of BE in subjects without, or with infrequent, reflux symptoms has been found to be substantial. In some studies, it is comparable with that of subjects with reflux symptoms. In one study, 8.3% of patients with GERD had BE, compared with 5.6% of patients without GERD. This finding adds to the challenge of finding the right patient population to target for screening. Relying on GERD as the sole risk factor for screening is also impractical because of the millions of subjects with frequent reflux symptoms. A better stratification of patients for screening is sorely needed.
Additional risk factors have been identified over the past few years, to help better delineate patients at risk. Central obesity (in particular visceral abdominal fat, measured by waist/hip ratio or waist circumference, rather than body mass index [BMI]) has been shown to result in increased esophageal injury and proinflammatory cytokine release, resulting in systemic inflammation and increased risk of developing BE and EAC. These associations have been found to exist independently of GERD. Other risk factors that are associated with BE are ( Table 1 ) male gender, non-Hispanic white ethnicity, older age, family history of BE or EAC, obstructive sleep apnea, and smoking. A combination of these factors likely increases the risk of BE and EAC in an additive or synergistic manner.
| Clinical Variable | Reference | Study Design | Sample Size | End Point | Results | Comments |
|---|---|---|---|---|---|---|
| Age | Guardino et al, 2006 | Cohort | 837 | EAC/HGD | OR of 3.5 for age ≥50 y with prevalent EAC compared with age <50 y | Rates of BE progression were similar in both age groups |
| Oberg et al, 2005 | Cohort | 140 | EAC/HGD | HR 1.062 (0.98, 1.16) | — | |
| Eloubeidi and Provenzale, 2001 | Case control | 211 | BE | Age ≥40 y independent predictor ( P = .008) | Prospective | |
| Edelstein et al, 2009 | Case control | 615 | BE | OR per decade for IM 1.3 (95% CI, 1.1–1.5) | — | |
| Johansson et al, 2007 | Case control | 764 | BE | Prevalence increased 5% by age (95% CI, 1–9) | Prospective study | |
| Cooper et al, 2014 | Case control | 3749 | EAC | OR, 1.03 (95% CI, 1.01–1.05); P = .005 | — | |
| Male gender | Oberg et al, 2005 | Cohort | 140 | EAC/HGD | HR, 1.062 (0.98, 1.16) | — |
| Yousef et al, 2008 | Meta-analysis | NA | EAC/HGD | Pooled estimate, 10.2 per 1000 person-years in men | Subgroup analysis of 6 studies included | |
| Edelstein et al, 2009 | Case control | 615 | BE | OR, 1.5 (95% CI, 1.1–2.2) | — | |
| Menke-Pluymers et al, 1993 | Case control | 158 | EAC | OR, 2.4 (CI not available); P = .06 | — | |
| Gerson et al, 2001 | Cohort | 517 | BE | Logistic regression analysis used to create a prediction model; P = .05 | Prospective nature. 7-symptom questionnaire | |
| Cook et al, 2005 | Meta-analysis | NA | BE | Pooled male/female ratio, 1.96:1 (95% CI, 1.77–2.17:1) | 32 studies | |
| Cooper et al, 2014 | Case control | 3749 | EAC | OR, 3.06 (95% CI, 1.50–6.24); P = .002 | — | |
| Ethnicity | Guardino et al, 2006 | Cohort | 837 | EAC/HGD | NA | Mainly white people in the study (76%) |
| Balasubramaniam et al, 2012 | Cohort | 1058 | BE | OR, 2.40 (95% CI, 1.42–4.03) | Prospective | |
| Length of BE | Guardino et al, 2006 | Cohort | 837 | EAC/HGD | NA | On multivariate analysis, length ≥3 cm resulted in 2.5× risk of developing EAC or HGD |
| Edelstein et al, 2009 | Case control | 615 | BE | OR for LSBE 1.4 (95% CI, 1.5–11.4) | — | |
| Yousef et al, 2008 | Meta-analysis | NA | EAC/HGD | Incidence in LSBE and SSBE was comparable (6.7 and 6.1/1000 patient-y respectively) | Subgroup analysis of 26 studies | |
| Sato et al, 2008 | Case control | 62 | EAC/HGD | Association between LSBE and EAC/HGD reported | Individual HRs not provided | |
| Rudolf et al, 2000 | Cohort study | 309 | EAC | Risk of EAC in SSBE similar to LSBE ( P >.2). If HGD on index EGD, no association of length and EAC risk | Prospective. Baseline dysplasia is an important factor to consider |
These findings have led to increased interest in creating risk scores for BE. Three such tools have been created, but their utility in clinical practice is unknown at this time. The initial tool, created in 2012, was externally validated and showed age, gender, highest level of education, smoking history, BMI, and use of acid suppressants as predictors of BE. The area under the curve (AUC) was 0.70 on the initial test model, and 0.60 for the independent validation dataset. In contrast, the AUC for using only GERD as a predictor of BE was 0.61 ( P <.001). The second tool is the Michigan Barrett’s Esophagus Prediction Tool. It is a model that predicts the likelihood of BE based on age, GERD symptoms, waist/hip ratio, and pack years of cigarette smoking. This model was generated after evaluating subjects undergoing screening colonoscopy with a research upper endoscopy for BE. All variables listed are equally weighted, and the AUC was 0.72. The third tool combined clinical (GERD symptom frequency and duration, age, gender, ethnicity, waist/hip ratio, and Helicobacter pylori status) and circulating cytokine markers (serum levels of interleukin [IL] 12p70, IL6, IL8, IL10, and leptin). The AUC for this model was 0.85, which was significantly improved from a GERD-only model, but external validation is still pending. These models give hope. Since the last cost-effective analysis was performed ∼12 years ago, there is interest in identifying the patient population that is best served by screening. Other than the broad categories mentioned earlier, there has been an interest in creating predictive tools.
Minimally invasive Barrett’s esophagus screening techniques
The most used modality for BE screening is sEGD. It is the current benchmark against which other tools are assessed. As mentioned previously, there are issues with accessibility, cost, and use. Some alternatives to sEGD have been explored, such as esophageal video capsule endoscopy (VCE), transnasal endoscopy (TNE), and noninvasive esophageal capsule cytology (cytosponge).
A population-based study evaluated the acceptability of VCE, TNE, and sEGD in a community population. It found that two-thirds of the population was interested in BE screening using noninvasive means. This finding is in contrast with a Veterans’ Affairs (VA) study that showed only 15% acceptability of VCE and TNE. However, in the VA study, patients had limited knowledge of BE and EAC. Nevertheless, in both studies, VCE was the preferred modality, although the difference was not statistically significant. The performance characteristics of VCE are currently suboptimal for BE diagnosis, with a meta-analysis quoting pooled rates of sensitivity and specificity as 77% and 86% respectively. In addition, the technique is not cost-effective, given current costs and need for confirmatory sEGD. Reduction of costs may render this technology cost-effective in the future. With potential advances in VCE technology (ability to zoom, addition of filter to change white light to blue light), it may be possible to improve diagnostic accuracy for BE.
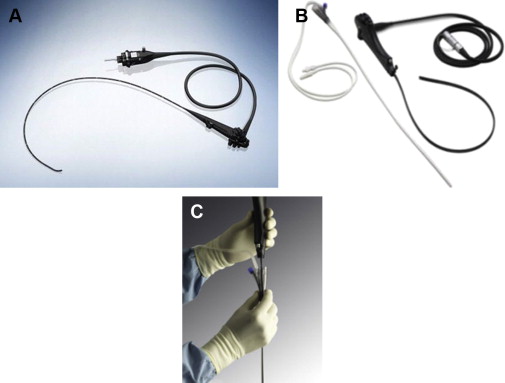
Another option for visualizing the esophagus is to use a transnasal endoscope (TNE). This device uses a thinner caliber scope (diameter, 5.4 mm vs 9.8 mm) and enters the esophagus via the mouth or nares ( Fig. 1 A). It has the benefit of being used without sedation and has accuracy for BE detection that is comparable with sEGD. Endoscopic assessment of BE and detection of intestinal metaplasia (IM) was equivalent between TNE and sEGD in a randomized trial from the United Kingdom. Tolerability was equivalent, with preference of TNE because of the lack of sedation and associated patient costs. Physician extenders can be successfully trained in the use of TNE for BE screening, with comparable accuracy and tolerability rates. An esophagoscope with a disposable sheath (Vision Sciences, Orangeburg, NY) with or without a biopsy channel has recently been developed (see Fig. 1 B, C). The sheath can be disposed of after a single use, with the endoscope ready for use without conventional disinfection. A recent randomized controlled trial found that the acceptability, quality, and yield of TNE performed in a hospital endoscopy unit (huTNE) and in a mobile research van (muTNE) was comparable with sEGD performed in a hospital endoscopy unit. TNE was well tolerated, with comparable yield (BE, esophagitis) and with substantially shorter time to complete esophageal evaluation.
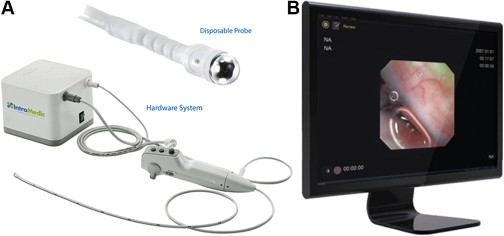
Another transnasal device (EG2 scan, Intromedic Inc, Seoul, Korea) is being investigated (NCT02066233) as a potential minimally invasive, unsedated technique for BE screening ( Fig. 2 ). This novel device has a probe capable of 30 frames per second with a 125° field of view. It is maneuverable along a single axis and is single use. The device has no suction or biopsy channel, but does allow insufflation with air. Initial tolerability, safety, and accuracy for the diagnosis of BE seem to be reasonable.
A nonendoscopic option being developed is esophageal capsule cytology, called the cytosponge. This device is the size of a pill and is ingested ( Fig. 3 ). It contains a compressed ball-shaped mesh on a string. The patient is asked to swallow the pill with the string attached. The string is held without any tension to allow the capsule to enter the stomach. Once it is inside the patient’s stomach, the gelatin layer dissolves to release the sponge (measuring 25 mm in diameter). As it is withdrawn from the patient, it brushes the gastroesophageal junction and esophageal epithelium to capture cells for analysis. The cytology specimen is then sent for histologic and immunohistochemical analysis. The cytology specimen is analyzed for trefoil factor 3 (TFF3), a recently identified marker of columnar epithelium. Compared with sEGD, the sensitivity of cytosponge/TFF3 assay is 73% and specificity 93.8% for BE segments greater than 1 cm. Sensitivity increases to 90% and specificity remains at 93.5% for BE segments greater than 2 cm. If validated in other cohorts, the cytosponge has the potential to replace sEGD, because it is noninvasive and easy to perform in the primary care setting. In a recent economic assessment, it was a cost-effective method to screen 50-year-old men with GERD for BE, versus no screening or screening with conventional endoscopy. If dysplastic BE or early EAC is detected by this method, it will be followed by sEGD and endotherapy for definitive management. One of the concerns with this technology was the low acceptability by patients (18%). Validation studies in other countries are currently underway, and may shed further light on the feasibility of this technique. The balloon cytology device is another nonendoscopic technique that has been studied. However, it has poor sensitivity (80% for high-grade dysplasia [HGD] but 22% for low-grade dysplasia [LGD]) and tissue sample acquisition (70%). Therefore, there is limited use for it in the current paradigm.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




