Fig. 8.1
Number of fecal O. formigenes with changes in dietary oxalate (■) or dietary calcium (□). Daily calcium intake was 1000 mg on the varied oxalate dietary phase and daily oxalate was 250 mg on the varied calcium dietary phase. Real-time PCR was used to quantitate O. formigenes numbers. 5.5 × 104 CFU/ng DNA was used to convert qPCR data to number of O. formigenes per g feces (Modified from Jiang et al. [19]. With permission from Elsevier)
Enumeration in stool suggests O. formigenes represents a tiny fraction of the total intestinal microbiota [23]. Many low abundance bacteria are thought to survive in the intestines by occupying specific nutrient niches where competition for their food source is limited [12]. Indeed, both in vitro culture studies [29] and a recent human study [19] show that O. formigenes utilizes oxalate more efficiently than many other bacteria. Thus, an important factor in the survival of this organism in the intestines is its unique ability to outcompete other bacteria for its food source.
In a recent study, total stool oxalate measurements showed O. formigenes colonized individuals excrete significantly less oxalate than non-colonized individuals when consuming diets controlled in their level of nutrients including oxalate and calcium [19] (Fig. 8.2), highlighting the highly efficient oxalate degrading capacity of O. formigenes relative to other microbiota. These data also show that the oxalate degrading capacity of the microbiome of non-colonized individuals is negligible at low oxalate intake, but increases with adaptation to ingestion of higher levels of dietary oxalate, as the dietary oxalate recovered in stool with a daily intake of 250 and 750 mg dietary oxalate was ~80 % and ~60 %, respectively. The impact of these “generalist” oxalate degrading bacteria in calcium oxalate stone disease is not known. Several human studies that have assessed the ability of bacteria with oxalate degrading potential to reduce urinary oxalate excretion have led to promising but generally mixed results. For example, the intake of commercial probiotic preparations, including Oxadrop® (VSL Pharmaceuticals), that contain a mixture of lactic acid bacteria known to degrade oxalate in vitro, reduced urinary oxalate excretion in patients with enteric hyperoxaluria [26] or after an oral load of oxalate [1]. However, when tested in healthy subjects consuming an oxalate rich diet [33], in patients with idiopathic hyperoxaluria [13], or patients with mild hyperoxaluria [27] probiotic supplementation did not reduce urinary oxalate significantly, suggesting chronic supplementation with “generalist” oxalate degrading bacteria may only be beneficial for patients with absorptive (enteric) hyperoxaluria.
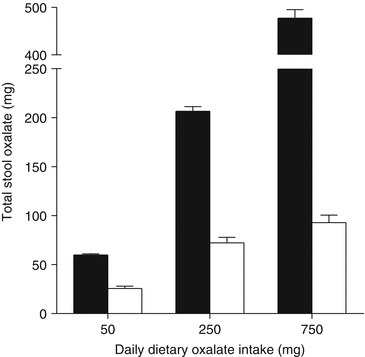

Fig. 8.2
Stool oxalate of O. formigenes colonized (□) and non-colonized healthy subjects (■) on nutrient controlled diets varying in oxalate. Daily calcium intake was 1000 mg (Modified from Jiang et al. [19]. With permission from Elsevier)
O. formigenes Colonization
Little is known about how and when individuals become colonized or how O. formigenes persists over time. The source of O. formigenes that colonizes the gut is not known. Studies to date suggest it occurs early in childhood [32] and based on what we know about O. formigenes transmission from animal experiments it is obtained from the environment, not directly from the mother [8].
A review of the colonization frequencies conducted worldwide indicated that 38–77 % of a normal population is colonized and it was consistently observed that the colonization frequency in stone formers was about half that in normal subjects [20, 21]. Several studies have indicated that the intake of antibiotics can result in the loss of colonization [21, 22, 25, 28], and this is supported by lower prevalence of O. formigenes in both cystic fibrosis patients [30], and calcium oxalate stone formers who are frequently prescribed antibiotics [28, 31]. It is also possible that a lower rate of colonization in stone formers is due to patients restricting dietary oxalate intake. To date, there has only been one study to examine factors that impact colonization, and in this study [21] only a slight (non-significant) trend was observed between prevalence of colonization (simply whether or not a person was colonized with O. formigenes) in normal subjects and oxalate intake. The impact of dietary oxalate deprivation on O. formigenes colonization warrants further examination.
The ability to re-colonize individuals lacking O. formigenes has previously been addressed by a study in which two healthy adults not colonized with O. formigenes became colonized following the ingestion of cultured O. formigenes [11], and subsequently remained colonized for 9 months. However, other studies where O. formigenes was provided in the form of an enteric coated capsule or as a frozen paste to patients suffering from Primary Hyperoxaluria, resulted in only a minority of the patients remaining colonized post-treatment [17, 18]. Therefore, although it seems quite possible that O. formigenes colonization of non-colonized stone formers may be a cheap and effective way to help minimize stone risk in calcium oxalate stone formers, long term colonization studies are required.
O. formigenes Colonization and Risk of Calcium Oxalate Stone Disease
Since the discovery of O. formigenes in 1985 and the recognition that it resides in the human gut and degrades oxalate, a role for the organism in stone disease has been considered. Initial case–control studies with small numbers of subjects suggested colonization may be protective against stone disease [6, 28, 35], as measurements of urinary oxalate excretion were lower in colonized compared to non-colonized individuals despite a large variability in oxalate excretion and a lack of dietary oxalate and calcium control during urine collections. In addition, a recent study showed 24 h urinary oxalate excretion and plasma oxalate were significantly lower in O. formigenes colonized patients compared to O. formigenes negative patients on a standardized diet [34]. Colonization was also found to be significantly inversely associated with the number of stone episodes.
Similarly, the association of recurrent calcium oxalate stone disease and a lack of O. formigenes was assessed in a study of 247 calcium oxalate stone formers and 259 matched controls [20]. The odds ratio for forming a recurrent stone when colonized was found to be 0.3, which indicates a 70 % reduction in stone risk. Surprisingly, there was no difference in urinary oxalate excretion between colonized and non-colonized individuals in either group, which may be due to highly variable oxalate excretion results despite a large enough sample size as well as the fact that dietary oxalate and calcium levels were not controlled. The discordance in results may be partially explained by our study in healthy subjects that illustrated that the beneficial oxalate degrading activity of O. formigenes is highly dependent on diet [19]. In this study, the most beneficial effect of O. formigenes was observed
when colonized subjects were administered a low calcium (400 mg/day) and moderate oxalate (250 mg/day) diet, urinary oxalate excretion was found to be lowest indicating that the efficiency of this bacterium is not maximal at all calcium and/or oxalate concentrations. Further controlled dietary studies are needed to examine what levels of dietary oxalate and calcium intake are required for successful colonization of non-colonized calcium oxalate stone formers with O. formigenes.
Interestingly O. formigenes has been demonstrated to induce gastrointestinal oxalate secretion in animal models, which may be a second mechanism by which this organism decreases oxalate levels within the circulation and the kidney [14–16]. A recent controlled dietary study with 11 O. formigenes calcium oxalate stone formers and 26 non-colonized calcium oxalate stone formers, showed absorption of a 13C2-oxalate load was not significantly different between the groups, but plasma oxalate concentrations were significantly higher in non-colonized (5.79 μmol/l) compared to O. formigenes colonized stone formers (1.70 μmol/l) [34]. These data support the findings in rodent models that O. formigenes induces enteric secretion of endogenously produced oxalate, thereby decreasing plasma oxalate concentration. Whether the modification of host oxalate transport properties by O. formigenes colonization underlies the reduction of risk for calcium oxalate stone formation is currently being tested by OxThera, Inc., in a Phase 2 clinical trial with Primary Hyperoxaluria patients.
Conclusions
Much still remains to be learned about how O. formigenes establishes and maintains gut colonization. Unraveling these mechanisms is especially important with respect to the colonization of non-colonized stone formers. Further studies on the factors involved in colonization resilience and enteric secretion of host derived oxalate are warranted in light of this. The range of conditions where O. formigenes lowers stone risk and the role the composition of the gut microbiome plays in this remain to be clearly defined.
Acknowledgements
The work from our laboratory was supported by NIH grants DK62284 and DK87967.
References
1.
Al-Wahsh I, Wu Y, Liebman M. Acute probiotic ingestion reduces gastrointestinal oxalate absorption in healthy subjects. Urol Res. 2012;40(3):191–6. doi:10.1007/s00240-011-0421-7.CrossRefPubMed
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




