Table of toxins and environmental factors affecting male fertility
Environmental and lifestyle factors
Mechanism of action
Effect
Reference
Agricultural influences
Fertilizers
Nitric oxide
Impaired spermatogenesis
Decreased sperm function parameters: motility, viability, acrosome reaction
[26]
Pesticides
Dichlorodiphenyltrichloroethane (DDT)
Impaired Leydig cell development, decreased testosterone receptors
Decreased testosterone levels, decreased sperm concentration
Ethylenedibromide
1,2-dibromo-3-chloropropane ethylenedibromide
[30]
Chlorpyrifos
Increased production of reactive oxygen species (ROS)
ROS-induced DNA damage and lipid peroxidation of spermatozoa
[31]
Arsenic
Genetic and epigenetic changes in the genome, carcinogenesis
Subfertility
[38]
Herbicides
Lindane
Increased production of ROS, damaged Sertoli cell morphology, impeded function of steroidogenic enzymes and proteins
Oxidative stress-induced DNA damage, decreased sperm counts and reduced circulating testosterone levels
Methoxychlor
Increased production of ROS
Oxidative stress-induced DNA damage, decreased sperm counts
Dioxin-TCDD
[33]
Vinclozolin
Acts as endocrine disruptor and androgen receptor antagonist, impaired embryonic testicular cord formation, increased apoptotic germ cell numbers
Impaired testes development, impaired spermatogenesis later in adult life, reduced sperm function
[39]
Industrial Influences
Toluene
Increased production of ROS, decreased antioxidant levels
Oxidative damage, reproductive toxicity and decreased sperm count and testosterone levels
Xylene
Inhibition of mitochondrial respiration and enhanced ROS production
Decreased sperm functional parameters
Acrylamide
Inhibition of sperm maturation and motility and increased inheritable DNA fragmentation
Spontaneous abortion and birth defects in the offspring
[30]
Perchloroethylene (PCE)
Impaired sperm function parameters
Prolonged conception timeframe and spontaneous abortion
[45]
Polychlorinated biphenyl (PCB)
Impaired spermatogenesis, increased gonadotropin-releasing hormone, decreased LH, antiandrogenic and antioestrogenic
Impaired sperm function, decreased testosterone levels
[46]
Epigenetics
Mutations in gametes and gamete production line
Transmission of genetic phenotypes between generations and possible harm to testes and subsequent seminal parameters of progeny
Cigarette smoke
Nicotine
Disrupted oxygen supply to tissues, induced inflammation and oxidative stress, impaired Leydig cell function
Lowered sperm concentration, declined motility and increased abnormal morphology, lowered male to female ratio of offspring, lowered testosterone levels
Hydroxycotinine
Nitrosamines
Carbon monoxide
Alkaloids
Polycylic aromatic hydrocarbon (PAH)
[9]
Nutrition, Obesity, Exercise
Vitamin C
Decrease in quality of diet, less intake of key antioxidants
Subfertility
Vitamin E
Selenium
[76]
Carbendazim
Inhibited steroidogenic- and antioxidant enzymes and increased production of H2O2-inducing oxidative stress in Leydig cells
Decreased testes weights, decreased sperm functional parameters, reduced seminiferous tube diameters
Parabens
Lowered oestrogenic activity and mitochondrial interaction
Possible role in unexplained male infertility
[94]
Alcoholism
Increased circulating levels of oestrogens, induced hypoxia, reduced FSH, LH and testosterone levels, oxidative stress, reduced antioxidants and lipid peroxidation
Impaired spermatogenesis, testicular atrophy, impotence, impaired libido, severely impaired seminal parameters
Radiation
RF-EMW
Stimulation of NADH oxidase, activation of leukocytes and generation of ROS, decreased melatonin, impaired Leydig cells, increased scrotal temperatures
Lipid peroxidation and oxidative stress, decreased cellular antioxidant levels, increased malonyldialdehyde (MDA) levels
X-ray radiation
Endocrine disruption and decreased sperm quality
Dose responsive subfertility, high dosages leading to irreversible sterility
Stress
Scrotal heat stress
Impaired spermatogenesis
Decreased sperm profile parameters and difficulties in achieving pregnancy
Influenza
Malaria
Varicocele
Cryptorchidism
Psychological, noise stress
Decreased antioxidants, increased free radical production, induced oxidative stress, increased glucocorticoids, decreased testosterone, lipofuscin accumulation in Leydig cells, decreased testosterone
Impaired male reproductive function, lowered semen quality
Gonadotoxins, chemicals and male reproductive system
Plastics
Plasticizers
Increased ROS production, increased H2O2 levels, antioxidant depletion, induced oxidative stress
Impaired sperm parameters and difficulties with reproduction
Bisphenol A (BPA)
Nonylphenol
Di (2-ethyhexyl) phthalate [DEHP]
Testicular atrophy, inhibition of spermatogenesis via ROS production and Zinc depletion
Heavy metals
Lead
Increased ROS synthesis and induced oxidative stress, inhibition of antioxidant enzymes
Impeded spermatogenesis and decreased sperm concentration
Cadmium
Antisteroidogenic: lowered testosterone secretion. Impaired Leydig cell function. Pro-oxidant: increased free radical production and reduced zinc levels. Disrupts inter-Sertoli cell tight junctions and impairs blood/testes barrier
Pharmacological agents
Sulfa-drugs
Impaired spermatogonia
Impaired spermatogenesis and long-term use can lead to infertility
Tetracyclines
Finasteride
Impaired spermatogenesis
Decreased sperm function parameters
Recreational drugs: marijuana, cocaine, methamphetamine
Endocrine disruption
Impaired male reproductive capabilities
[196]
Anabolic steroids
Impaired LH secretion, impaired testosterone production, impaired spermatogenesis
Oligozoospermia, hypogonadotropic hypogonadism
Phases of Male Reproductive Development and Environmental Insult
Male reproductive development starts in utero during gestation and starts with the initiation of testes development from the bipotential gonad. The bipotential gonad differentiates from the genital ridge, which forms as a thickening of somatic cells on the surface of the mesonephros from which it originates. After gonadal determination structures develop dependent on hormone regulation and this process is known as sexual differentiation. The gonad gives rise to three bipotential cell lineages responsible for the formation of Sertoli cells, steroidogenic cells and cells responsible for completion of gonadal structural development. The first foetal precursor cell lineage is responsible for the formation of steroidogenic cells responsible for the secretion of sex hormones and secondary sexual characteristics. The second cell lineage gives origin to Sertoli cells and mesenchymal cells. The Sertoli cells regulate the synthesis of the seminiferous tubes, while the mesenchymal cells differentiate into Leydig cells. The third cell lineage differentiates into the gonad structure. Development of the bipotential gonad is dependent on the anti-Mullerian hormone secreted by the Sertoli cells, testosterone secreted by interstitial cells and the insulin-like 3 hormone. The intermediate mesoderm is homologous for male and female development and gives rise to the Wolffian ducts, Mullerian ducts and the gonad precursors. During male development, the Mullerian duct, under influence of testosterone, dissolves away while the Wolffian duct gives rise to the epididymis, vas deferens, ductus deferens, ejaculatory duct and the seminal vesicle. Thus, the precursor cells and subsequent spermatogonia are of cardinal importance to spermatogenesis in the adult male. It is crucial that precursor cells proliferate unimpeded and give rise to an optimal amount of spermatogonia. The development of the external male genitalia is dependent on dihydrotestosterone. The transfer of the testes from the genital ridge to the scrotum is a process of cardinal importance to sexual differentiation. Testosterone induces the relaxation of the cranial suspensory ligaments allowing the descent of the testes into the scrotum. The increased abdominal pressure due to the viscera growth and the elastic properties of the testes then cause the testes to be forced through the inguinal canal and into the scrotum. After the initial development of the essential male reproductive organs, the reproductive system lies dormant until puberty when the hypothalamic-pituitary gland (HPG) axis becomes active and the process of spermatogenesis can initiate.
With the onset of puberty the hypothalamus secretes gonadotropin releasing hormone (GnRH), which stimulates the anterior pituitary causing the secretion of the gonadotropins : follicle stimulating hormone (FSH) and luteinizing hormone (LH) . The LH is responsible for the stimulation of testosterone and thus the onset of secondary sexual characteristics. The FSH is responsible for the stimulation of Sertoli cells responsible for the onset of spermatogenesis [6−8].
Spermatogenesis is the process whereby the mature male reproductive system produces haploid gametes from diploid spermatogonia during a complex and delicate process that initiates in puberty and continues right through a male’s lifetime. Spermatogenesis requires a combination of synchronized gene expression and cell division and takes place in the testes over a period of a little more than 2 months.
Of cardinal importance to the normal occurrence of spermatogenesis are the Sertoli cells as they alter rates of sperm production in adult testes and produce factors essential to gamete development [9−11]. Leydig cells are responsible for the secretion of androgenic hormones. These androgens are key to appropriate testicular development, such as urethral groove fusion and descent of the testis [12, 13].
Due to the intricacy of the process, spermatogenesis is totally dependent on the existence of optimal conditions. It is extremely sensitive to changes in the external environmental elements. Therefore, environmental insult that affects gonadal differentiation, Sertoli- or Leydig-cell proliferation or spermatogenesis at any age could affect male reproductive development and thus lead to adverse reproductive pathologies such as oligozoospermia, asthenozoospermia, hypospadias, testicular spermatogonia cancer and cryptorchidism [7−9].
The male reproductive system can be exposed to adverse environmental factors during any of its three stages of development: maternal-dependent (gestation and lactation) development, early-life (prepubertal)—development or sexual maturity (Fig. 12.1). Specifically, the effects of environmental factors during the two developmental stages (maternal/early-life) can be detrimental to testicular development and spermatogenesis. This can result in poor semen parameters later in life, including impaired sperm concentration and motility. There can also be direct exposure to hostile environmental factors during adulthood. Similar to the maternal and infancy stages of development, such factors can have a negative impact on spermatogenesis. However, direct exposure during later life is regarded as reversible while early life exposure is considered to be irreversible [14, 15]. Overall exposure to adverse elements can impair the male reproductive system during any of its stages of maturity and thus affect spermatogenesis through several mechanisms of action:
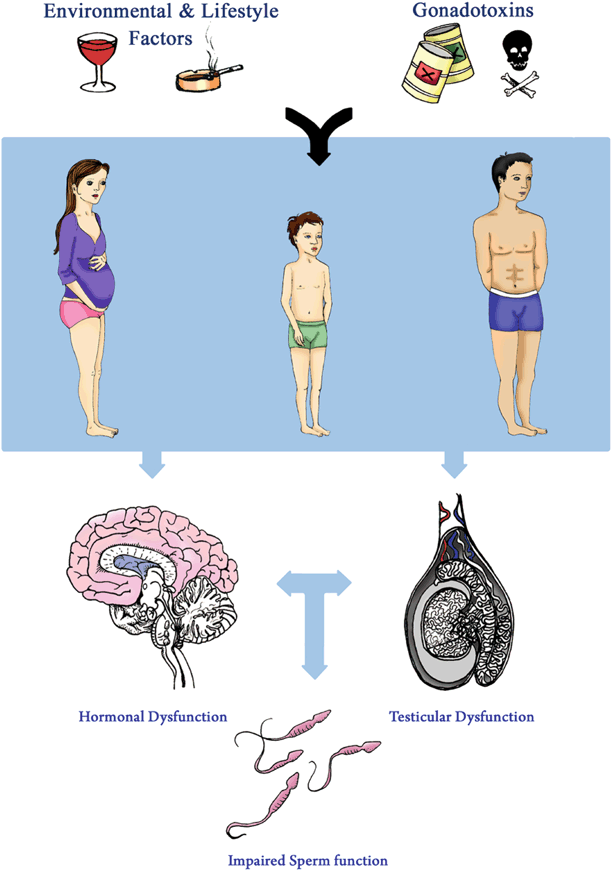

Fig. 12.1
Effects of environmental factors, lifestyle factors and gonadotoxins on male fertility. Exposure of male reproductive system to environmental factors, lifestyle factors and gonadotoxins during gestation, early life and adulthood could affect hormonal and testicular regulation, thus contributing to impaired sperm function and associated male infertility
Any stage of maturity: Impediment of spermatogenesis and/or Sertoli- and Leydig-cell function can affect spermatogenesis in later life.
Maternal exposure: Endocrine-inhibiting substances and adverse lifestyle factors can affect reproductive organ development.
Postnatal exposure: Environmental fluctuations such as changes in scrotal temperatures can affect spermatogenesis in later life.
Adulthood exposure: Harmful substances such as xenobiotics or adverse lifestyle factors can affect spermatogenesis.
Lifestyle factors , such as cigarette smoking during pregnancy, have proven to reduce sperm concentration in developing males as well as Sertoli cell count. This is because the components in cigarette smoke antagonize androgen receptor-mediated function and thus impede reproductive organ development. Maternal obesity has also been shown to reduce sperm concentration in the male offspring and inhibit testicular development via interference of the foetus’ testosterone/oestrogen balance. Diet of the mother can also affect the developing foetus through, for example, ingestion of anabolic steroids found in meat. These anabolic steroids and the oestrogenic substances used to process and cook the meat can act as xenobiotics and impair the critical hormone balance in foetal development leading to impaired spermatogenesis in the mature offspring. Other harmful substances such as herbicides and pesticides, which are lipophilic, can also be absorbed and start amassing in the fat of pregnant mothers. These substances are then slowly released to the foetus and infant via placental uptake and breast-feeding [16−19].
Fluctuations in postnatal thermal scrotal temperatures may lead to an adverse reproductive state known as scrotal heat stress that is responsible for a decline in sperm count in later years (as discussed in section ‘Heat, Noise and Psychological Stress’). Studies have shown that the use of disposable plastic-lined diapers instead of reusable cotton diapers during infancy and early childhood induce higher scrotal skin temperatures [20−22].
Environmental insults in the form of oestrogens have been shown to cause responsive changes in the neuroendocrine system of the mature male with effects notable in reproductive function and spermatogenesis [23]. Such environmental oestrogens, known as xenobiotics, can have a negative impact on male fertility as ingestion of these substances has been directly correlated to decreased sperm concentration. Xenobiotics have been found to adversely affect the male reproductive system in the following ways:
The inhibition of FSH secretion by the foetal pituitary gland and thus a disturbance in the HPG-axis leading to a decreased number of Sertoli cells
The inhibition of Leydig cell formation and function leading to decreased testosterone production and decreased gamete differentiation
The inhibition of androgen receptors within foetal testes
The conversion of xenobiotics to quinones that produce reactive oxygen species (ROS) that, when produced in excess, induce oxidative stress or damage DNA [24].
Environmental Factors, Lifestyle Factors and Male Infertility
As already noted, it is increasingly evident that spermatogenesis is an immensely sensitive and delicate process that is dependent on optimal conditions and severely susceptible to fluctuations in external factors [7]. The rapid expansion of Western lifestyle with its concomitant increase in industry, changes in diet, excessive alcohol consumption, bad smoking habits and high levels of stress may be responsible for the aforementioned decrease in male fertility.
Agricultural Influences
With a world population of just under 7 billion people, sufficient food production has become a major worldwide issue, giving rise to biotechnology and food engineering/modification as a whole new form of industry.
While the introduction of fertilizers, herbicides and pesticides has made large-scale food production possible, it has also introduced a new set of chemicals and possible toxins that could adversely affect a great number of people globally. Nitrogen and ammonia are two currently used fertilizers in food cultivation and can stimulate nitric oxide (NO) production. When found in excessive levels in the body, NO, inhibits spermatogenesis with a resultant drop in sperm motility, viability, acrosome capacitation and ability to fertilize the egg [25]. Pesticides such as dichlorodiphenyltrichloroethane (DDT), 1,2-dibromo-3-chloropropane, ethylenedibromide, vinclozolin and organophosphates have proved to inhibit sperm concentration and studies have shown that farmers who have high exposure to such pesticides have a higher incidence of infertility than men in other occupations [26−29]. Another organophosphate pesticide, chlorpyrifos, has been implicated in ROS-induced DNA damage and lipid peroxidation of spermatozoa [30]. Herbicides such as lindane, methoxychlor and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) have all been directly correlated to oxidative stress and decreased sperm concentration. Lindane exposure is also detrimental to the reproductive system by damaging the morphology of Sertoli cells, resulting in decreased spermatogenesis. Lindane exposure also impedes the function of steroidogenic enzymes, steroidal regulatory and transport proteins and thus reduces circulating testosterone levels [31−36].
The large-scale use of such fertilizers, herbicides and pesticides is proving to be of major concern to fertility specialists. Eradicating the use of these agents is probably not a realistic solution to the problem as the agents address the much more basic human need for food on which millions of lives depend.
Industrial Influences
In industrial environments substances commonly occur that have been found to adversely affect the fertility of males that regularly work in these environments. Examples of such substances are toluene, xylene, acrylamide and perchloroethylene (PCE).
Toluene is an organic solvent found in paint, rubber, glue, gasoline and several cleaning agents. It is ingested through the inhalation of vapour. Studies have shown toluene to decrease epididymal sperm concentration and decrease testosterone levels by inducing a state of oxidative stress through the excess production of ROS or a decrease in the antioxidative capacity of cells [27, 37]. Another substance commonly found in industrial areas, Xylene, has presented in workers’ blood and semen where it has been found in the air in excess of the allowable atmospheric amount. Studies have shown xylene to decrease sperm viability, motility and acrosin activity by inducing a total impediment of mitochondrial respiration and stimulating mitochondrial ROS production [37−41]. Acrylamide, known as an intermediate in the production of polyacrylamide, is used in water purification, paper production and mining. Acrylamide has been associated with the inhibition of sperm maturation and motility and increased inheritable DNA fragmentation. PCE is a substance found in the cleaning industry and has been found to prolong conception timeframes and even cause spontaneous abortion in the partners of men exposed to it [42].
As industry expands, better health and safety regulations should be put in place to protect the health of workers. Fertility specialists should do a full history check and test for industrial substances when attempting to treat men working in this sector who are experiencing fertility issues.
Epigenetics
Transgenerational inheritance is a term that refers to the ability of environmental factors to not only promote a pathophysiologic condition in an individual but to promote it in successive generations. Most environmental parameters such as nutrition or toxins do not directly cause DNA mutations or alterations in DNA sequence but do have the ability to alter the epigenome. Mutations in the gametes and gamete production line, which become irreversible, can cause transmission of genetic phenotypes between generations and can cause downstream harm to the testes and subsequent seminal parameters of the progeny. Transgenerational and early life exposure to adverse environmental factors, such as endocrine disruptors, are now considered key factors in the onset of adult reproductive impediment. Imprinted genes are specific genetic factors dependent on epigenetic programming that can be influenced by environmental factors such as nutritional factors, inorganic toxins such as arsenic, endocrine interrupters such as bisphenol A (BPA), phytoestrogens and chemicals used as fungicides and pesticides [43−54].
Cigarette Smoke
The adverse effects of smoking on the body and on male fertility are well-established—yet cigarettes remain one of the world’s top selling commodities. Smoke inhalation has been correlated to lower sperm concentration, declined motility and increased abnormal morphology in sperm. Smoking disrupts the oxygen supply to tissues and the reproductive system as well as exposing the reproductive system to more than 2000 substances that have the potential to harm, such as nicotine, carbon monoxide, nitrosamines, alkaloids and hydroxycotinine. These substances can increase the production of free radicals such as ROS and reactive nitrogen species (RNS), which when produced at pathophysiologic levels, can lead to oxidative stress and ultimately to infertility [55−58]. Studies have found a direct correlation between cigarette smoke intake, increased ROS levels and decreased levels of antioxidants . Studies have also correlated smoking with an increase in cadmium levels. Cadmium is a heavy metal known for its detrimental effects on reproduction [56, 58]. It is understood that metabolites that enter the circulation as a result of smoking act as chemotactic stimuli-inducing inflammation and attract leukocytes that produce ROS and induce oxidative stress [56, 59]. Smoking has proved to decrease seminal levels of antioxidants such as vitamins C and E [60, 61] and has been associated with higher levels of tetrazoospermia [62].
Tobacco smoking has also been shown to reduce the ratio of male to female offspring, even if only the father smokes [63]. The nicotine and cotinine found in smoke either inhibit the intracellular calcium content or completely block the effects of calcium on steroidogenesis in Leydig cells, resulting in a decline in circulating testosterone levels [64]. In addition, nicotine intake results in increased blood cholesterol levels causing atherosclerosis in arteries supplying blood to the reproductive system and thus resulting in lowered blood [65]. Chronic exposure of the male reproductive system to nicotine leads to a state of vasoconstriction or vasospasm within the penile arteries and smooth muscle leading to an impairment of Leydig cell function [66, 67].
Nutrition and Exercise
When assessing nutritional factors and their effects on the male reproductive system, there are three factors to take into account:
Diet and malnutrition
Obesity
Ingestion of preservatives
With the spread of Westernized lifestyle in modern times, many cultures have moved away from their traditional diets and conform to new eating habits by consuming more refined carbohydrates and less fresh fruit and vegetables. Fruits and vegetables contain essential nutrients such as antioxidants, vitamins and folate that play key roles in DNA and RNA synthesis during spermatogenesis. Many of these nutritional elements are also antioxidants of paramount importance. Vitamins C and E are both antioxidants that neutralize ROS and prevent oxidative stress. Vitamin C protects the sperm DNA in the seminal fluid and Vitamin E protects the spermatozoa membranes. Selenium is a mineral that also functions as an antioxidant. Studies have reported that these antioxidants improve male fertility parameters when either used alone or in combination. Studies have shown that a decrease in ingestion of these nutritional substances is correlated to subfertility [27, 68−73].
With an increased intake of refined carbohydrates, lipids and proteins as associated with increased global Westernization; overweight and obese individuals have become an ever-growing issue affecting the health and economic status of many countries. Men with a body mass index (BMI) of over 25 are up to three times more at risk to be classified as infertile due to reduced sperm concentration and increased sperm DNA fragmentation.
There are three main theories that attempt to explain the link between obesity and infertility. First, studies have shown a direct correlation between change in BMI trends and changes in endocrine and exocrine functions of the testes. The prevalence of excess adipose tissue leads to the conversion of testosterone to oestrogen, thereby decreasing the levels of circulating testosterone and increasing the levels of estradiol . These changes are accompanied by decreases in LH and FSH, leading to impaired spermatogenesis. Second, accumulation of inner thigh, pubic and abdominal fat could cause infertility through increased scrotal temperatures. Third, obesity and many of its associated conditions such as dyslipidemia and insulin resistance , which all form part of the broader condition known as metabolic syndrome, are associated with induced states of systemic proinflammation. Systemic proinflammation is accompanied by increased activation of leukocytes, production of ROS and subsequent onset of oxidative stress and lipid peroxidation, which severely impair sperm parameters [74−85].
Preservatives found in food and other consumer products as broad spectrum antifungal agents (such as fruit, paint and textiles) can be harmful to male reproductive parameters. Studies have correlated carbendazim (methyl-2-benzimidazole carbamate) intake to decreased testes weight, low sperm concentration and motility, reduced seminiferous tube diameters and increased incidence of abnormal sperm. Researchers postulate that carbendazim adversely affects reproductive systems by inhibiting steroidogenic and antioxidant enzymes and increasing production of hydrogen peroxide (H2O2) radicals. These changes then induce oxidative stress and cause lipid peroxidation in Leydig cells [86−89]. Recent research done on preservatives that have been commonly used and regarded as safe for many years, such as parabens (alkyl esters of p-hydroxybenzoic acid), has concluded that these substances may not be as safe as previously thought. Such substances may interact with mitochondria and so play a role in unexplained infertility [90−92].
Alcohol
Evidence suggests that moderate alcohol consumption does not have any effect on male reproductive function . Excessive chronic alcohol use, however, does harm spermatogenesis and male fertility. Alcoholism has been associated with testicular atrophy, impotence, impaired libido, reduced FSH, LH and testosterone levels, oxidative stress, reduced antioxidants and lipid peroxidation and severely impaired seminal parameters. Studies suggest that alcohol adversely affects the reproductive system by promoting the overproduction of free radicals such as ROS, and inducing a state of oxidative stress in the testes as well as inducing hypoxia and causing tissue damage in a system already very sensitive to changes in oxygen supply. Alcoholics often follow diets that deprive them of antioxidants [93−99].
Excessive alcohol intake also causes an increase in circulating levels of oestrogens in males. The increased oestrogen levels disrupt the normal production of testosterone and cause saturation of testosterone receptors in the hypothalamus in the brain. This in turn leads to a reduced signal sent to the pituitary gland, which in turn reduces the secretion of luteinizing hormone (LH) . Ultimately, this results in reduced testosterone production in the gonads. An increase in circulating oestrogen also increases the production of sex hormone-binding globulin (SHBG). The SHBG binds testosterone and reduces the plasma levels of testosterone. Testosterone levels in plasma are key to the homeostasis of the gonadotropins (LH and FSH), which regulate spermatogenesis and the maturation of sperm cells [100, 101].
Cell Phone, Laptop and Ionizing Radiation
With the increasing modern day usage of electronics that transmit electromagnetic waves, much attention has been drawn to the possible effects of such devices on the human body. Studies have shown that cell phone usage directly correlates to a decline in male fertility parameters. Cell phones transmit via radiofrequency electromagnetic waves (RF-EMW) that significantly increase malonyldialdehyde (MDA) levels (a lipid product of lipid peroxidation and oxidative stress) and decrease cellular antioxidant levels. Researchers postulate that cell phone-associated radiation can lead to the induction of oxidative stress through either
the stimulation of the sperm plasma membrane redox system, which entails activation of NADH oxidase, or
the activation of leukocytes and the subsequent generation of ROS.
Cell phone-associated radiation also seems to decrease melatonin, which acts as an antioxidant in the body. Other morphological research reports that RF-EMW emitted from cell phones adversely affects Leydig cells via a thermal molecular mechanism resulting in impaired spermatogenesis. Electromagnetic field (EMF) exposure was directly correlated to a decline in seminiferous tubular diameter and epithelium thickness [102−110].
Exposure of ionizing radiation in chronic doses has also been associated with endocrine disruption and decreased sperm quality. Studies done on cooperating prisoners opting for testicle X-ray irradiation, as well as men exposed to radiation after the Chernobyl nuclear tragedy, reported a direct correlation between dose of ionizing radiation and increasing infertile parameters with high dosages of exposure even leading to irreversible sterility [111−115].
Laptops wirelessly connected to the Internet through Wi-Fi, transmit via RF-EMW radiation and, when used on a person’s lap, increase scrotal temperatures. A recent pilot study shows that ex vivo exposure of human spermatozoa to RF-EMW via an Internet-connected laptop decreases motility and increases DNA fragmentation. This suggests that laptops connected to the Internet, used on the lap in close proximity to the testes, could impede male infertility, but here more research is needed [116].
Heat, Noise and Psychological Stress
Exteriorization (descending) of the testes is unique to mammals. It is an evolutionary adaptation to keep the testes at a core temperature of 3–4 °C cooler than the normal 37 °C internal abdominal temperature. Keeping the testes cool reduces rates of DNA damage and resulting sperm mutations. Any fluctuations in the testes temperature, caused for, e.g. by occupations, lifestyle choices or disease, can disrupt this susceptible system of homeostasis and impair spermatogenesis [117−119].
Occupational chronic exposure of the body to raised temperatures has been correlated to decreased sperm profile parameters and difficulties in achieving pregnancy. This problem is especially prevalent in people with occupations that entail working in close proximity to a furnace, for example welders, bakers and stokers. Some studies, however, still contest these findings and thus the effect of chronic heat exposure remains a somewhat controversial subject. Prolonged periods of sitting and inactivity associated with office jobs have been shown to correlate with high scrotal temperatures and decreased sperm concentration. Sperm density has been directly correlated to a decrease by 40 % per 1 °C change in the daytime scrotal temperature. Professional drivers have also been associated with reduced seminal quality and difficulties in achieving pregnancy [120−129].
Lifestyle choices can have an effect on scrotal heat temperatures and subsequent infertility. Wearing tight fitting underclothing such as briefs as opposed to wearing looser fitting boxers can cause increased scrotal temperatures. Prolonged hot baths, steam rooms and saunas increase scrotal temperature to such an extent as to inhibit spermatogenesis. As previously mentioned, dietary choices leading to obesity can increase scrotal temperature and thus impede spermatogenesis [130−137].
Illnesses such as influenza or malaria and conditions such as varicocele or cryptorchidism could also lead to heightened scrotal temperatures. Studies have shown a decrease in seminal quality after the onset of fever caused by influenza or malaria [138−140].
Situations causing a subject to experience mental stress have been associated with impaired male reproductive function . Mental stress has been associated with lower levels of antioxidant enzymes and higher levels of oxidants possibly leading to an induced state of oxidative stress. Studies have also shown a correlation between mental stress and lowered semen quality. For example, a study done on students showed that semen quality was severely affected by stress caused by exams. It is well-established that stress leads to increased levels of glucocorticoids, but it is also associated with decreased levels of testosterone [141−145]. Corticosterone administration is known to stimulate free radical production and lipofuscin formation in the mitochondria of Leydig cells. Similarly, studies show that chronic noise stress can lead to lipofuscin accumulation in testes and decreased testosterone levels. This means that chronic noise stress could prove to be detrimental to male reproductive systems [146−148].
Studies that provide a good example of the effect of both mental and noise stress on the male reproductive system examine the effect of war on the reproductive system. Studies on the 15-year Lebanese civil war have reported that infertile males had a 57 % correlation to civil war-related trauma (residence in bombing areas, participation in combat, injuries, kidnapping and displacement from home). There was also a significant association between war involvement and decreased sperm concentration and increased abnormal sperm morphology [149, 150].
Gonadotoxins and Male Infertility
It is inevitable that, with spermatogenesis proving to be such a susceptible process, chemical exposure as a result of environmental pollution will lead to adverse sperm parameters and difficulties with reproduction [111]. Over the past 50 years, average sperm concentrations in the general population have decreased by 50 %. During this same period extensive environmental and lifestyle changes have occurred in both First and Third World Countries. There has been astronomic growth in the chemical industry, with an ever-increasing market for new products leading to ever-increasing masses of product waste [151, 152]. Studies in this field battle with the immense difficult of identifying and analysing the effect of a single external factor such as environmental pollution on a complex organism which is constantly exposed to a mixture of toxins and environmental insults.
Plastics
Plastic has become a very common substance in consumer products found in most households all over the world. Plastic suppliers often add substances when producing plastics to make them more functional and useful. Many of these added substances are, however, quite toxic to the reproductive system. Plasticizers are polyphenolic chemical substances used to prolong the elasticity and durability of plastics and are common in clear, heat-resilient and indestructible plastics. Plasticizers have been found to be harmful to the male reproductive system. Another chemical commonly found in plastics is bisphenol A (BPA). BPA is an additive to disposable plastics used to improve polycarbonate plastics; it is also used in dental materials. BPA has been found to have the ability to migrate from the plastic of food containers into food and from dental sealants and fillings into circulation in the human body. BPA has been found to be present in the blood of 90 % of Americans. BPA has proved to inhibit sperm concentration, motility and viability. Studies have shown that BPA generates ROS in several tissues, including the reproductive tissues, and causes an increase in the levels of H2O2 in testicular tissue. This increase in free radicals ultimately leads to a depletion in cellular antioxidant defences, an imbalance in oxidant–antioxidant production and a subsequent induced state of oxidative stress [153−157]. Phthalate esters have been used to increase the elasticity of plastics in bags, toys, clothing and pharmaceutical products such as soaps and shampoos. Animal studies report that a specific phthalate ester, Di(2-ethyhexyl) phthalate (DEHP), causes testicular atrophy and inhibition of spermatogenesis via ROS production and zinc depletion, but the effects in humans are still in dispute [158−163]. Nonylphenol is a synthetic constituent of plastic that has oestrogen-like properties and is lipophilic and is therefore capable of crossing cell membranes and concentrating in tissue. Nonylphenol is often found in cleaning chemicals , paints, pharmaceutical products, foods and in certain forms of packaging materials. Adult exposure to nonylphenol has been correlated to decreased sperm concentration [91, 164].
Heavy Metals
Studies show a direct correlation between heavy metal exposure and fertility implications such as impeded spermatogenesis and decreased sperm concentration [165−168]. Lead and cadmium are two key metals of concern to male infertility. Lead has been banned from most products by governmental legislation but was previously found in products such as lead paint and gasoline leading to occupational overexposure. Lead can also accumulate in fish and be ingested when eating fish. Inorganic lead can disturb the oxidant–antioxidant balance and induce a state of oxidative stress. Lead inhibits the delta amino levulanic acid synthase enzyme and thus promotes ROS synthesis and induces oxidative stress. Lead has also been associated with inhibited antioxidant enzyme activity through the impediment of superoxide dismutase, catalase and glutathione peroxidase activity [169−172].
Cadmium has been found to be directly connected to male fertility problems. Cadmium levels are higher in the seminal plasma and blood of infertile men than that of fertile men. Cadmium affects the male reproductive system in several ways. It directly inhibits sperm concentration due to its antisteroidogenic properties that lead to a lowered testosterone secretion. It directly impedes the function of Leydig cells and thus the testosterone levels. It has been found to have pro-oxidant properties and may mediate generation of free radicals and reduction in zinc levels, zinc being crucial to spermatogenesis. Finally, cadmium may disrupt inter-Sertoli cell tight junctions and thus disrupt the blood/testes barrier and consequently inhibit spermatogenesis. Chronic smoking has been associated with significantly increased cadmium levels [173−176].
Other metals such as zinc, copper, aluminium, mercury and vanadium are under scrutiny for their possible adverse effects on male fertility [177]. More research is of paramount importance, but there is sufficient evidence that heavy metal exposure is harmful to the male reproductive system. Metal workers and other men who are occupationally exposed or exposed through lifestyle choices that seek infertility treatment should have a full heavy metal assessment as part of their diagnosis and treatment course.
Pharmacological Agents
Since pharmacological agents have become more readily available on mainstream markets the human body has been bombarded by a whole range of pharmacological agents. These agents have often not been properly investigated as to their effects of long-term use on reproductive and other tissues. Drugs in long-term use whether prescription, recreational or muscle enhancing have been associated with possible adverse effects on the male reproductive system. Therapeutic drugs such as antibiotics and chemotherapy can impede the function of spermatogonia. Antibacterial drugs such as sulfa-drugs and tetracyclines can impair spermatogenesis and long-term use can lead to infertility. Studies have shown that men that ceased use of common chronic drugs such as allergy, epilepsy and bacterial infection treatment drugs showed a 93 % improvement in semen profile. Drugs used to treat reproductive conditions such as androgenic alopecia and benign prostatic hyperplasia such as finasteride, impede spermatogenesis and further decrease sperm function parameters. Such studies all state that the type of drug, dosage and duration of use are factors that contribute to the effect on the male reproductive system. All studies, however, conclude that such drugs affect the male reproductive capabilities [178−184].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







