Low-risk ERC
High-risk ERC
Absolute factors
Morphology
– Polypoid
– Ulcerated
– Sessile
– Flat raised
Tumor grade
G1–G2
G3–G4/signet ring
Depth of invasion
Haggitt 1–3
Haggitt 4
T1sm1
T1sm2–3
Lympho-vascular invasion
No
Yes
Resection margin
R0
Rx or R1
Relative factors
Tumor budding
−
+
Mucinous histology
−
+
Position in distal 1/3 rectum
−
+
Tumor size
<3–4 cm
>3–4 cm
Cribriform-type structural atypia
−
+
All patients who are surgical candidates for per anal excision, TEM, or TAMIS are similarly suitable for robotic transanal excision. Neoplasms in the upper, mid, and lower rectum can all be removed utilizing a robotic transanal approach; however, this approach is optimal for tumors in the range of 6–10 cm from the anal verge. Although very low-lying lesions, whose distal margin sits below the edge of the access channel, may be amenable to robotic excision, one would have to strongly consider the benefits or advantages of this.
Perhaps the most valuable indication for robotic transanal surgery would be in the treatment of complex benign disorders such as rectourethral fistula or rectal anastomotic revisions that would benefit from the magnified three-dimensional image and tremor-free intuitive instrument movement to perform accurate hemostatic dissection with improved suturing capabilities (Video 16.1). However, while robotic transanal surgery (RTS) is in its development, it is more appropriate that less intricate cases are chosen at the start of a surgeons’ learning curve, i.e., benign mid-rectal polyps or excision of a scar following the endoscopic removal of a malignant polyp (Video 16.2).
16.3 Robotic Approach for Resection of Rectal Neoplasms
All patients who have been selected to undergo RTS resection must have also undergone colonoscopy to assess for synchronous lesions and to obtain a biopsy of the rectal lesion.
A pragmatic approach suitable for most practices is to assess the primary tumor clinically and then radiologically with rectal ultrasound and increasingly now MRI to identify those with unexpected advanced T-stage and more importantly evidence of nodal involvement. On this basis an early rectal cancer most suitable for local excision should be <3 cm in diameter, freely mobile, ultrasound stage T1, and well or moderately differentiated on biopsy, lack lympho-vascular invasion and mucinous architecture, and be free from nodal disease on MRI. A significant proportion of rectal lesions treated by local excision are only confirmed as malignant on the final postoperative pathology. This can make staging of the mesorectum later difficult as a result of regional postsurgical changes both in the wall and potentially lymph nodes. Avoidance of this situation is critical; the threshold for preoperative imaging by MRI or ultrasound even in benign-appearing lesions should be low, especially when high-grade dysplasia is present by biopsy [27]. Carcinoembryonic antigen level (CEA) and CT body imaging is also performed to assess for tumor metastasis. Patients with stage IV disease or locally advanced lesions are not candidates for robotic transanal surgery unless the objective is palliation.
Preoperative preparation is according to institutional guidelines; broadly speaking patients receive bowel preparation the day before the planned surgery to ensure minimal contamination of the operative field, in particular if peritoneal entry is anticipated. Preoperative antibiotics are administered prior to the initiation of surgery and standard deep venous thrombosis prophylaxis is undertaken.
All procedures are performed under general anesthesia; a peripheral nerve block around the anus consisting of 20-mL 0.5 % bupivacaine can be administered to aid in relaxation of the sphincters and improve postoperative analgesia.
Patient positioning is not dictated by tumor location with various setups having been reported irrespective of this. For obvious reasons the prone position avoids any conflict of the robotic arms with the patients’ lower extremities. While lithotomy and the lateral approach are less cumbersome and provide better access to the airway for the anesthesiologist, there is the potential for conflict of the robotic arms, particularly in obese patients. In lithotomy position particularly, docking requires strategically moving the legs and stirrups around the robotic arms.
While the robotic setup might seem cumbersome and time-consuming, the docking time significantly reduces with experience [28–30]. In the cohort recently reported by Hompes et al., the mean docking time for the last five cases was less than half of the first five cases (22 min vs. 52 min, respectively), and it is the authors’ opinion that the setup will not be a major impediment to the use of the robot for transanal procedures (Fig. 16.1) [31].
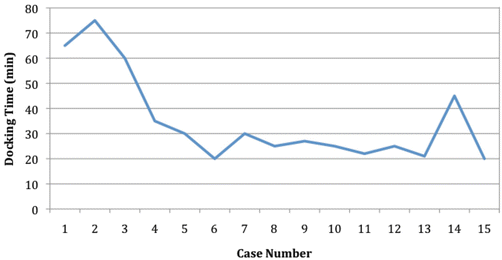

Fig. 16.1
Demonstration of the learning curve for the docking procedure. With permission from Hompes R, Rauh SM, Ris F, Tuynman JB, Mortensen NJ. Robotic Transanal Minimally Invasive Surgery for local excisions of rectal neoplasms. Br J Surg 2012;101:578–81. © Blackwell Science in 2012 [28]
To date all robotic intraluminal cases have been performed through a GelPOINT path (Applied Medical Inc., Rancho Santa Margarita, CA, USA) or transanal glove port. The GelPOINT path consists of a 4-cm long access channel with a 4 cm diameter, combined with a gel cap seal and 3–4 working 12-mm ports. The transanal glove port is a novel less-expensive creation utilized for transanal access where an airtight channel is created using an Alexis retractor (Applied Medical), plastic anoscope, and sterile surgical glove with trocars fabricated through the digits. The setup of the glove port is demonstrated in Video 16.3. Interestingly, the glove port may facilitate the robotic setup, enabling flexibility by permitting docking of the cannulas away from the limited perianal workspace. Furthermore, the glove port allows for a wide axis of movement for instruments inside the rectum enabling them to be used more widely apart or easily rotated and/or crossed. It is this latter feature which is of particular interest for the robotic approach. The crossed setup for the cannulas with switched robotic arm control allows for additional intraluminal reach while still maintaining completely intuitive control. Also, inherent to this setup is the maximal separation of the robotic arms externally reducing collision between the robotic arms and/or camera. We have found that external conflict was more common in proximal lesions and with the use of the 5-mm robotic instruments, since they lack the robotic EndoWrist® technology, and the crossed setup proved particularly helpful to eliminate this. The benefit of the 5-mm instruments is their narrower profile, which allows for easy transition from a crossed setup to a parallel setup without any help from the bedside assistant (Video 16.4). Furthermore, the elbows of the joints can help to stent the rectal lumen in case of an unstable pneumorectum or to get access to a lesion proximal to a rectal valve of Houston.
After introduction of the transanal platform, the robot (da Vinci® Si Surgical System, Intuitive Surgery, Sunnyvale, CA, USA) is side-docked with the forks of the robotic cart parallel with and lining up with the caudal base of the table (Fig. 16.2, Video 16.3). A pneumorectum of 10–15 mmHg is established using a standard laparoscopic carbon dioxide insufflator. The robotic instruments are introduced into the rectum under direct vision (Video 16.1); the robotic cannulas can be positioned in parallel or crossed (with reversed arm control assignments) depending on the height and location of the lesion within the rectum. Standard setup entails the use of an 8.5-mm robotic 30° camera in various positions; however, the 30° upward camera positions in our experience circumvent collisions of the robotic arms, in particular with the parallel setup. With the 30° upward camera positions, insertion of the camera through the thumb of the surgical glove (“thumbs-up” position of the camera) avoids excessive strain on the glove material and avoids tearing of the glove (Fig. 16.3a, b). A variety of 8-mm EndoWrist® instrumentation or 5-mm instrumentation can be used, including monopolar permanent cautery hook, Maryland bipolar forceps, DeBakey forceps, and a small or large needle driver. A bedside surgeon provides endoluminal assistance with a laparoscopic grasper, disposable suction-irrigation device, or scissors.
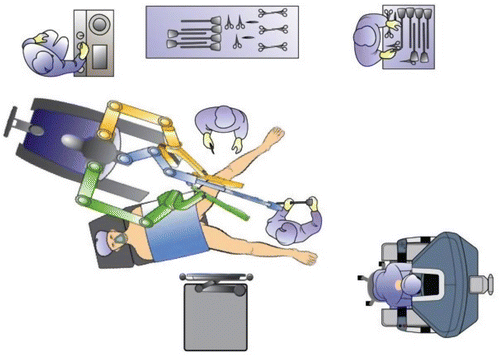
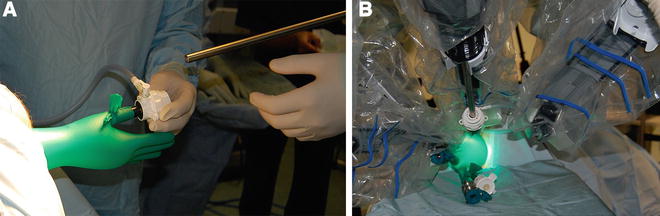

Fig. 16.2
Room setup and docking position for the robot and patient for RTS performed in the lithotomy position

Fig. 16.3
(a) 12-mm camera port is inserted through the thumb of the glove (“thumbs-up position”), and after establishing a pneumorectum, the access to the rectum is assessed. (b) After docking of the camera, two 8-mm ports are inserted into the tips of two fingers
The technique of robotic transanal resection is identical to resection performed with other advance platforms. A robotic 5-mm hook cautery and Maryland grasper initially are placed in the two operating trocars. The console surgeon then performs a full-thickness local excision, first by demarcating the perimeter of the lesion with generally a 1-cm margin using monopolar energy. For evacuation of smoke, a bedside assistant uses a 5-mm laparoscopic suction-irrigator device passed directly through the GelPOINT cap without the need for a trocar or through the fourth finger of the glove. Simple short bursts of suction maintain image clarity without collapsing the rectal lumen. The specimen may be tented gently using a robotic Maryland grasper, while hook cautery allows for full-thickness excision. Importantly, the CO2 insufflation provides a natural “pneumo-dissection,” thereby augmenting the ease and clarity of local excision using robotic transanal surgery. To retrieve the resected specimen, the robot must be dismounted from the GelPOINT path interface. The lesion can be retrieved with a 5-mm grasper, the lid to the port simply removed allowing for specimen extraction. When the glove port is used, the specimen can be stored in the pouch of the glove and retrieved at the end of the procedure.
Closure of the full-thickness rectal wall defect, which is generally recommended, is then performed. The hook cautery and Maryland grasper are exchanged with one or two robotic needle drivers. Robotic intraluminal suturing is then carried out using a V-Loc 180 absorbable wound closure device (Covidien, Mansfield, MA). This allowed for suturing without the need for intraluminal knot tying, since the unidirectional barbs on the suture self-lock as they pass through the rectal wall. The defect can be closed with a single running V-Loc stitch, thereby completing the operation (Videos 16.1 and 16.2).
As with any new technique, it is imperative that the quality of the resection is not compromised and safety of the patient is ensured. In the authors’ experience, the patients had comparable outcomes compared to the benchmarks set with transanal endoscopic surgery, and procedures were effectively and safely undertaken. While the postoperative follow-up is short, to date no local or distant recurrences were found.
Clearly there is still room for improvement of both the robotic instruments and transanal platform. Future optimization of this technique including the ideal setup (crossed vs. parallel) and type of instrumentation (8 mm vs. 5 mm) for the various lesion locations within the rectum and new developments in robotic platforms will increase its application for patients with advanced rectal lesions, offering rectal preserving therapy. Again while we describe here the use of the transanal robotic platform for local excision of rectal tumors, the stability and intraluminal versatility of this platform will lend itself perfectly to more advanced transanal procedures, transanal total mesorectal excision (taTME) perhaps being one of them.
16.4 Surgical Approach for Robotic Transanal TME
taTME is a newer technique used to facilitate dissection of the middle and lower third of the rectum. Although it is still in its infancy, with no long-term data, the approach seems to have many advantages over both open and laparoscopic rectal resection. Early data has consistently shown high negative circumferential margin (CRM) rates in addition to high-quality complete mesorectal specimens [16–18]. Robotic-assisted transanal surgery for total mesorectal excision (RATS-TME) is performed with a hybrid approach, relying on laparoscopic assistance for colon, upper rectum, and splenic flexure mobilization in addition to pedicle ligation. The abdominal approach can be performed prior to the initiation of the transanal approach; however, many choose to start the operation from below. The legs are elevated in Allen stirrups to the high-dorsal lithotomy position and the perineum is then prepped and draped appropriately. For low-lying lesions, the approach begins under direct vision and an intersphincteric resection is performed prior to robotic docking. The distal rectum is then closed with a running continuous suture of 2–0 Prolene. Alternatively, standard total mesorectal excision can be initiated with the access channel secured into the rectum from the outset and a Prolene purse string suture placed just above the anorectal ring. The gel cap is secured to the access channel and CO2 insufflation is turned on. Generally, transanal TME can be performed with the insufflator set at 8–10 mm of pressure, as the volume is smaller to fill as compared to the robotic transanal excision as described above. For robotic-assisted transanal TME, patients are positioned in moderate Trendelenburg, and the lower extremities are then returned to the low, modified lithotomy position to allow the robotic cart to be docked without encroachment. The robotic approach and cart docking is identical to that previously described. A 30° 8-mm HD robotic camera lens and 2 working arms—a 5-mm hook-monopolar cautery and a 5-mm Maryland grasper—are used for the dissection and these instruments are docked through the GelPOINT transanally. A bedside assistant again can utilize a laparoscopic suction-irrigator device mainly aiding smoke removal.
Working in a retrograde fashion from the distal rectum proximally, the mesorectum is dissected circumferentially maintaining the surgical tenants of total mesorectal excision. During the dissection of the distal 5 cm of rectum, the working space is limited and movement of the robotic arms fairly constrained. As the dissection progresses, however, there is usually an increase in the working space within the pelvis, and with the aid of pneumo-dissection, robotic taTME is significantly easier particularly in the mid-rectum. The upper rectum is more challenging to dissect using RATS-TME, as working angles necessary to complete the dissection are difficult to obtain, especially posterior.
As with the approach from above, the posterior and then lateral planes along the pelvis are established first, saving the anterior dissection for last. If the anterior dissection is carried out first, the rectum will be drawn posteriorly by gravity, making the posterior dissection extremely difficult.
Specimen extraction and anastomosis can all be performed transanally or for bulkier specimens through a Pfannenstiel incision or potential ostomy site if one is planned for temporary fecal diversion.
16.5 The Evidence for Robotic Transanal Surgery: Local Excision and taTME
The use of the robot endoluminally through a transanal approach only became feasible in 2009 with the development of TAMIS and the first approved flexible platforms designed specifically for transanal access (GelPOINT path, Applied Medical). Clinical data therefore remains limited. Further hindering the numbers lies in the inherent difficulty and limitations of the current robotic setup, which may offset the perceived benefits of increased endoluminal dexterity.
Atallah was the first to pursue robotic transanal surgery in 2010, first in a dry-lab setup and shortly thereafter in cadavers using variable approaches to docking ultimately settling on a parallel-docking approach with the patient in lithotomy position [29, 30]. Hompes and Mortensen in 2012 described an effective, cheap, and potentially improved technique through the adaptation of a transanal “glove port” for robotic access and subsequently published the largest series of robotic transanal surgery with 16 cases in May 2014 [31]. Various other case reports from the USA and Europe similarly have shown feasibility in a small number of patients (Table 16.2) [29, 30, 32–41]. No short- or long-term data exists regarding patient outcomes, recurrence rates, quality of resection, or survival. However, one would hope that outcomes equivalent to other advanced transanal platforms could be demonstrated, as this belies the principles of its intended purpose: to improve the ease, visualization, and quality of resection through the use of robotic micro instrumentation. Ultimately superior outcomes will be required to justify the increased cost of this technique, amounting to 1000 € (US$1700) per case in Hompes’ paper.
Table 16.2
Current published series of robotic transanal surgery
Author | Date | Country | PLAT | PROC | Model | n | BEN | MAL | DAV | SIZE | MRG | MES | LN | DOCK | OT | LOS | FU | Remarks
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|




