Robot-assisted Gastrectomy with Lymph Node Dissection for Gastric Cancer
Woo Jin Hyung
Yanghee Woo
Kazutaka Obama
Introduction
Robotic surgery for gastric cancer is increasing. Many surgeons have adopted robotic surgery to facilitate the technically challenging procedure of gastrectomy with D2 lymphadenectomy. With robotic gastric cancer surgery training, experienced laparoscopic surgeons can safely provide the advantages of minimally invasive surgery to their patients. Adherence to the oncologic principles of gastric cancer treatment ensures that the long-term survival benefits of surgery will not be compromised.
The indications for robotic surgery are similar to those of the conventional laparoscopic approach to gastric cancer. Early gastric cancer patients without perigastric lymph node (LN) involvement are ideal candidates for robotic gastrectomy with limited lymphadenectomy. Locally advanced gastric cancer without evidence of distant metastases is a generally accepted indication for robotic gastrectomy and D2 lymphadenectomy.
Indications for robotic gastrectomy with limited lymphadenectomy:
cT1N0M0
Mucosal and submucosal tumors not eligible for endoscopic resection
Failed endoscopic mucosal resection or endoscopic submucosal dissection
Indications for robotic gastrectomy requiring D2 lymphadenectomy:
cT1N1M0
cT2N0M0; cT2N1M0
Currently, there is no evidence to support robotic surgery for gastric cancer with serosal involvement (T4a) or invasion of adjacent organs (T4b), or for palliative intent. Intolerance to pneumoperitoneum is a contraindication.
The preoperative work-up of patients undergoing robotic surgery for gastric cancer requires complete evaluation of the patient’s clinical status, confirmation pathologic diagnosis, and estimation of the location and extent of disease. The preoperative work-up will guide each step of the surgical decision-making process.
Upper endoscopy with biopsy and with or without clipping proximal to the lesion
Endoscopic ultrasound
CT scan of the abdomen
Pertinent Anatomy
Robotic gastrectomy and lymphadenectomy requires the knowledge of gastric vessels and the accompanying nodal stations as defined by the Japanese Gastric Cancer Association. The operative procedure is described relative to the dissection of the LN stations in D2 lymphadenectomy.
Operating Room Configuration
The operating room configuration is centered on the patient and the da Vinci Surgical System (Sunnyvale, CA, USA). Relative position of the operating table, the surgeon console, the anesthesia cart, the surgical cart, the assistant, the monitors, and the robot during robotic gastrectomy are described.
The robot system is positioned cephalad to the patient.
The patient-side assistant is positioned to the lower left side of the patient on the opposite side of the scrub nurse, scrub table, and the main assistant monitor.
The vision systems rack is placed at the foot of the operating table.
The surgeon’s master console is positioned to grant the surgeon a view of the patient.
Patient Positioning, Port Placement, Robot Docking, and Preparation of the Operative Field
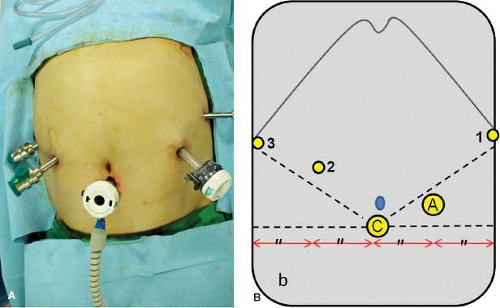
The patient is placed under general anesthesia, positioned supine with both arms tucked to the patient side, and urinary catheter is placed. The abdomen is prepared from the nipple line to the suprapubic region and draped in the standard sterile fashion. Five ports, two 12 mm and three 8 mm, are used for robotic gastrectomy (Fig. 21.1). Port placements may require minor adjustments for the patient’s body habitus. Once the ports are placed, the robot surgical cart is brought in from the head of the patient, and the robot arms are docked.
The camera arm is docked to the infraumbilical port (C)
The first arm holds the curved bipolar Maryland forceps
The second and the third arms hold the ultrasonic shears or a monopolar device and the Cadiere forceps, interchangeably.
Liver Retraction
The self-sustaining retraction of the left lobe of the liver is required during robotic gastrectomy as in other upper abdominal surgeries. Adequate liver retraction is a prerequisite for complete dissection of the suprapancreatic lymphadenectomy and along the lesser curve of the stomach. Several methods have been described.
Intraoperative Tumor Localization to Determine the Resection Extent
Intraoperative tumor localization is required to determine the appropriate margin of resection during robotic subtotal distal gastrectomy. Since robotic surgery is performed for lesions without serosal involvement, the lesion cannot be readily detected during the operation. Intraoperative tumor localization has been achieved by several different methods including dye injection, intraoperative endoscopy, or laparoscopic ultrasound. A successful technique using preoperatively placed endoclips and an intraoperative abdominal x-ray is a simple and effective method.
Procedure of D2 LN Dissection During Distal Subtotal Gastrectomy
Five Steps and Associated Anatomic Landmarks
Partial omentectomy and left side dissection of the greater curvature: left gastroepiploic vessels
Right side dissection of the greater curvature and duodenal transection: head of pancreas and right gastroepiploic vessels
Hepatoduodenal ligament dissection and approach to suprapancreatic area: right gastric artery, proper hepatic artery (PHA), portal vein (PV), and celiac axis
Exposure of the root of the left gastric artery (LGA) and skeletonization of the splenic vessels
Lesser curvature dissection: esophageal crus and cardia; proximal gastric resection
Partial Omentectomy and Left Side Dissection of the Greater Curvature
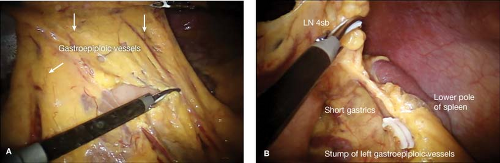
The exposure of the omentum can be achieved by creating a draping of the greater omentum for safe division and retrieval of LN stations 4sb and 4d (Fig. 21.2A).
Divide the greater omentum from the midtransverse colon toward the lower pole of the spleen.
Carefully identify, ligate, and divide the left gastroepiploic vessels at their roots. (Fig. 21.2B).
Clear the greater curvature of the stomach from the proximal resection margin to the short gastric vessels.
Right Side Dissection of the Greater Curvature and Duodenal Transection
Attention is directed to the right side of the patient for mobilization of the distal stomach from the head of the pancreas and dissection of the soft tissues containing LN
station 6 which is bordered by right gastroepiploic vein (RGEV), anterior superior pancreaticoduodenal vein (ASPDV), and the middle colic vein (Fig. 21.3).
station 6 which is bordered by right gastroepiploic vein (RGEV), anterior superior pancreaticoduodenal vein (ASPDV), and the middle colic vein (Fig. 21.3).
Release the connective tissues between the pancreas and the posterior stomach and the duodenal attachments to the colon.
Dissect the soft tissues on the head of the pancreas to identify, ligate, and divide the RGEV as it joins the anterior superior pancreaticoduodenal vein. (Soft tissues anterior to and superior to the ASPDV and superior to the middle colic vein should be retrieved on either side of the RGEV.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree