This article reviews the neurologic conditions associated with a high prevalence of bladder dysfunction and about which significant advances in understanding have occurred in recent years. The importance of the frontal lobes for bladder control has been confirmed through functional brain imaging, and recent findings in the elderly with incontinence suggest the problem may result from disconnection of important frontal areas caused by white matter disease. The very different urologic profile of the two sometimes-confused conditions, multiple system atrophy and Parkinson’s disease, is clarified. The advances in treatments for multiple sclerosis in recent years have been remarkable and are briefly described.
Many different neurologic pathologies can affect the central and peripheral nervous system resulting in neurogenic incontinence. A list of these is given in Box 1 and in depth discussion of these conditions can be found in standard text books. However, this article attempts to update the urologist on recent developments that have altered the neurologic understanding of a select group of diseases that have a particularly high incidence of bladder dysfunction.
Suprapontine causes
Stroke
Traumatic brain injury
Degeneration
Parkinson’s disease
multiple system atrophy
Alzheimer’s disease
Hydrocephalus, normal pressure hydrocephalus
Cerebral palsy
Neoplasm
Infrapontine-suprasacral causes
Demyelination
Multiple sclerosis
Transverse myelitis
Trauma
Vascular
Arteriovenous malformations
Spinal cord infarction
Neoplasm
Metastasis
Primary
Hereditary
Hereditary spastic paraparesis
Infections
Tropical spastic paraparesis (HTLV-I)
Spina bifida
Infrasacral causes
Cauda equina damage
Diabetes mellitus
Hereditary
Hereditary motor sensory neuropathy
Pelvic surgery
Frontal lobe function and disease
Although frontal lobe lesions have been known to produce bladder function disturbances since the publication of Andrew and Nathan in 1964, recent findings based on functional brain imaging techniques have greatly enhanced understanding and knowledge of the importance of this brain region for bladder control. Based on an analysis of both the positron emission tomography (PET) and functional MRI (fMRI) studies, a working model of lower urinary tract control by higher brain centers was proposed in a recent review article, an illustration from which is shown in Fig. 1 .
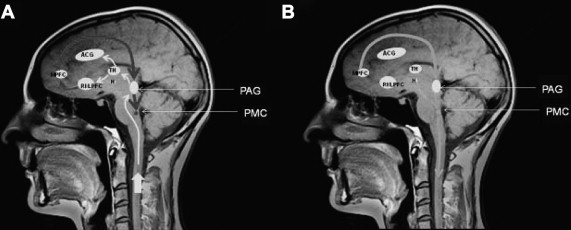
As anticipated from the results of animal experimental studies, the primary relay center for bladder afferents is now known to be a midbrain structure, the periaqueductal gray (PAG). This region has rich connections with pelvic organs, and it has been proposed that it has an important role in homeostasis and reproductive function. Activation of the PAG has now been shown in many studies of the effect of bladder filling, as illustrated by a recent meta-analysis of the literature by Griffiths and Tadic Fig. 2 .

The pontine micturition center (PMC) (see Figs. 1 and 2 ), formerly known as Barrington’s nucleus , is the brainstem region that connects directly to the sacral spinal cord, and its activation effects relaxation of the striated urethral sphincter followed by contraction of the detrusor muscle. The existence of an additional brain stem region, the L-region (see Fig. 2 ), activated during withholding micturition, is less clear. Current theory maintains that the PMC in humans does not receive direct bladder afferent input, but rather is “informed” by the PAG. The PMC is held in a state of inhibition by the PAG throughout bladder filling while continence is maintained through social considerations. Consciousness about the appropriateness of voiding is provided by input from higher centers, specifically the medial prefrontal cortex and the hypothalamus, with this input modulating PAG activity.
While inhibiting micturition, higher cerebral regions are also activated by bladder filling, and the right insula is particularly recognized as being an important structure in the process of “interoception.” Interoception can be defined as “the sense of the physiologic condition of the entire body,” and recently Craig emphasized that this includes the sensations of pain and temperature and visceral sensations. From the insula, activity is processed in the lateral prefrontal cortex and therefore acquires “hedonic valency,” or a qualitative value as to pleasantness or otherwise. The anterior cingulate cortex (ACC) is part of the limbic system, a network of brain regions involved in the processing and generation of emotions. The ACC is now thought to have an important role as the efferent system for autonomic activity.
Aging and incontinence
Symptoms of an overactive bladder and urgency incontinence have been shown to have an increasing prevalence with aging by many different studies, such as SIFO in Europe and NOBLE in the United States. Why this should be and what is the cause of the high incidence of incontinence in the elderly has long been uncertain. Although incontinence has long been known to be multifactorial, observational studies had shown that the disorder in the frail elderly was often associated with a degree of cognitive and functional decline and a tendency to falls, and therefore several risk factors seemed to be associated with incontinence, many unrelated to the genitourinary tract. However, recent advances in this area have shown that white matter lesions, formerly considered an incidental finding on MRI and of little clinical relevance, are not as benign as previously thought, but may instead be a significant factor in causing disconnections in brain areas critical to bladder control, such as those shown in Fig. 1 . White matter hyperintensities (WMH) causing leukoaraiosis , or white matter wasting, are commonly present on the MRI scans of older people and have been linked to vascular risk factors.
The association between WMH and urinary incontinence in the elderly was first recognized by Sakakibara and Hattori 1999. This Japanese group observed that although detrusor overactivity was found in 82% of elderly subjects with these MRI changes, it occurred in only 9% of those without. Furthermore, in those with mild leukoaraiosis, urinary incontinence was more common than was cognitive impairment or gait slowness, suggesting that urinary dysfunction is a common and early sign in elderly people with leukoaraiosis or white matter disease. These observations were subsequently confirmed and the hypothesis developed that the white matter damage involves focal tracts connecting centers involved in bladder control (see Fig. 1 ). Using computer-based imaging techniques, both to identify connecting white matter tracts ands quantify the amount of white matter disease, the severity and degree of bother from incontinence was shown to be associated with a high burden of WMH in the right inferior frontal region and specific white matter tracts that connect frontal regions to other brain regions involved in maintaining continence.
Aging and incontinence
Symptoms of an overactive bladder and urgency incontinence have been shown to have an increasing prevalence with aging by many different studies, such as SIFO in Europe and NOBLE in the United States. Why this should be and what is the cause of the high incidence of incontinence in the elderly has long been uncertain. Although incontinence has long been known to be multifactorial, observational studies had shown that the disorder in the frail elderly was often associated with a degree of cognitive and functional decline and a tendency to falls, and therefore several risk factors seemed to be associated with incontinence, many unrelated to the genitourinary tract. However, recent advances in this area have shown that white matter lesions, formerly considered an incidental finding on MRI and of little clinical relevance, are not as benign as previously thought, but may instead be a significant factor in causing disconnections in brain areas critical to bladder control, such as those shown in Fig. 1 . White matter hyperintensities (WMH) causing leukoaraiosis , or white matter wasting, are commonly present on the MRI scans of older people and have been linked to vascular risk factors.
The association between WMH and urinary incontinence in the elderly was first recognized by Sakakibara and Hattori 1999. This Japanese group observed that although detrusor overactivity was found in 82% of elderly subjects with these MRI changes, it occurred in only 9% of those without. Furthermore, in those with mild leukoaraiosis, urinary incontinence was more common than was cognitive impairment or gait slowness, suggesting that urinary dysfunction is a common and early sign in elderly people with leukoaraiosis or white matter disease. These observations were subsequently confirmed and the hypothesis developed that the white matter damage involves focal tracts connecting centers involved in bladder control (see Fig. 1 ). Using computer-based imaging techniques, both to identify connecting white matter tracts ands quantify the amount of white matter disease, the severity and degree of bother from incontinence was shown to be associated with a high burden of WMH in the right inferior frontal region and specific white matter tracts that connect frontal regions to other brain regions involved in maintaining continence.
Bladder symptoms in movement disorders
Multiple System Atrophy
Multiple system atrophy (MSA) can present either as a poorly levodopa-responsive parkinsonism (MSA-P) or a cerebellar dysfunction (MSA-C), but in either condition, additional bladder dysfunction causing urinary incontinence is an early feature. The differential diagnosis between MSA-P and the more common disease, Parkinson’s disease, can be difficult even for neurologists who specialize in movement disorders. However, early troublesome incontinence and even earlier erectile dysfunction in men are now regarded as sinister warning signs that the neurologic condition may be the more rapidly fatal condition of MSA. Frequency occurring during the day and night, urinary urgency, urgency incontinence, incomplete bladder emptying, urinary retention, or a combination of these disorders can occur, with the expected adverse effect on quality of life.
Urodynamic investigations in patients with MSA commonly show detrusor overactivity as the underlying cause of overactive bladder symptoms. Detrusor overactivity is thought to result from the central pathology of MSA, which includes neuronal loss of neuromelanin-containing cells in the nigrostriatal dopaminergic system, cerebellum, pontomedullary raphe (resulting in the MRI appearance of the pontine cross sign), and frontal cortex.
Incomplete bladder emptying worsens with progression of the illness. A study measuring the postvoid residual volume in patients with MSA found a steady increase in mean postvoid residual between the first and fifth years. Incomplete bladder emptying is now recognized as being so characteristic of the bladder disorder in MSA that the finding of a raised postvoid residual volume has been suggested as a useful discriminator in the differential diagnosis of MSA and Parkinson’s disease. The neurologic basis for this finding has yet to be fully elucidated, but it may be related to the MSA disease process affecting the descending spinal pathways to the sacral parasympathetic outflow.
The combination of detrusor overactivity and incomplete bladder emptying contribute to the prominent urinary incontinence characterizing this condition. Continence is further compromised by the development of an open bladder neck and weakness of the striated urethral sphincter. The bladder neck receives its sympathetic innervation from the hypogastric nerve, and the pathology of MSA commonly affects the interomedial lateral cell columns in the thoracic cord that convey descending sympathetic innervation, resulting in deficits that underlie the postural hypotension and the open bladder neck found in this condition. In one study, an open bladder neck at the start of bladder filling without the accompanying bladder overactivity was found in 53% of patients with MSA but not in any with Parkinson’s disease ( P <.01). An open bladder neck may be asymptomatic in women but, in combination with the other disorders of bladder function seen in MSA, contributes to incontinence.
Denervation of the striated sphincters is an abnormality that is fairly specific for MSA, resulting from loss of anterior horn cells in the Onuf’s nucleus of the sacral spinal cord. This group of cells, first described by Onufrowicz in 1900, projects to the external sphincters and was found to show a selective loss in postmortem studies of patients dying with MSA. The striated urethral sphincter is critical for the maintenance of continence, and sphincter weakness in MSA may result in continuous urinary incontinence in female patients and contribute to poor bladder control in both sexes.
As the anterior horn cells of Onuf’s nuclei are spared in Parkinson’s disease, sphincter electromyogram (EMG) was proposed as a valuable means for distinguishing MSA-P from Parkinson’s disease, although others have not found the technique valuable. The value of sphincter EMG as a test to distinguish MSA from Parkinson’s disease has been greatly debated, but in a patient with a history of a cerebellar or akinetic rigid syndrome of less than 5 years and with significant urinary symptoms, a normal result makes the diagnosis of MSA unlikely.
Parkinson’s Disease
In recent years there has been increasing recognition that Parkinson’s disease involves many brain regions other than the substantia nigra and that many of the non-motor symptoms of the condition reflect this multisystem involvement. Although many different agents are effective for treating the motor manifestations of this condition, such as levodopa and dopamine agonists, these do little to ameliorate the non-motor symptoms, such as those listed in Box 2 .
Bladder disturbances
Nocturia
Urgency
Incontinence
Gastrointestinal disturbances
Dysphagia
Vomiting
Constipation
Fecal incontinence
Sexual Dysfunction
Erectile dysfunction
Reduced libido
Behavioral and cognitive changes
Depression
Anxiety
Memory loss
Poor concentration
Delusions
Sleep Disturbances
Hypersomnia
Insomnia
Vivid dreams
Rapid eye movement behavior disorder (enacting dreams)
Restless leg syndrome
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






