Fig. 13.1
Physiology of voiding
Ultimately, in order to properly empty, the bladder needs to generate a contraction strong enough to overcome the resistance of the outlet. Problems with this coordination, either caused by dysfunction of the bladder due to a reduction in the strength and/or duration of a detrusor contraction (detrusor underactivity), the outlet due to increased resistance or obstruction (bladder outlet obstruction), or both can cause incomplete bladder emptying.
Pathophysiology of Incomplete Bladder Emptying
Dysfunction of the outlet causing obstruction has been well studied. Chronic outlet obstruction has been associated with many changes to the morphology of the bladder, including changes to the extracellular matrix, electrical gap junctions, and smooth muscle enzymes and mitochondria. Obstruction has been shown to lead to a rapid hypertrophy of the bladder smooth muscle and an increase in collagenous connective tissue deposits, but with decreased myosin concentration, which ultimately results in a decreased force of contraction. It is theorized that obstruction results in acute ischemia, thereby affecting all components of the bladder that embody its viscoelastic properties (epithelium, connective tissue, vasculature, and smooth muscle), resulting in acute muscle dysfunction. The degree of dysfunction is thought to be related to the degree of tissue hypertrophy and not necessarily the duration of the obstruction. However, the bladder has amazing ability to regenerate and has shown evidence of recovery as early as fourteen days after obstruction.
Primary dysfunction of the bladder without obstruction, on the other hand, is not as well understood. A number of changes have been found to impact the bladder secondary to aging, ischemia, and comorbidities. Diabetic neuropathy has been shown to affect the innervation to the bladder, dull the sensory input, and induce changes to the physiology of detrusor smooth muscle. In cases of chronic overdistention, there can also be subsequent reversible or irreversible changes to the detrusor muscle cells. Studies focusing on the effect of aging on the bladder have demonstrated decreased ratios of detrusor muscle to collagen and changes in the quantity of muscle and collagen with aging.
A number of theories exist to try to explain the development of bothersome symptoms from incomplete bladder emptying. It has been postulated that the detrusor is organized into circumscribed modules controlled by a peripheral myovesical plexus, interstitial cells, and intramural ganglia. Bladder outlet obstruction may cause reduced blood flow, causing a transient or permanent ischemia that then affects sensitive nerve terminals and leads to denervation to certain modules. Supersensitivity then develops in the affected muscle modules, causing a reflexive excitation. Eventually the denervation progresses to an extent that the detrusor no longer functions and decompensates. These secondary changes that occur may explain why treatment will not always cause a resolution of symptoms. Other studies looking at smooth muscle proteins Connexin 43 and 26, the most predominant subtypes in the bladder, have found that increased fluid pressure from urine retention caused four- to fivefold increase in their levels, noticed as early at 7–9 h after obstruction. There have also been found to be increases in alpha-1 adrenergic receptor subtypes, particularly subtype alpha-1 d, now being identified as having a marked role in the development of irritative symptoms that are associated with bladder outlet obstruction.
Definitions Related to Urinary Retention and Bladder-Emptying Disorders
It is important to emphasize the value of having a common understanding of the terminology for purposes of reporting results and developing guidelines for treatment. The International Continence Society produced a report in 1988 to standardize definitions for a variety of lower urinary tract symptoms and conditions, which has subsequently been updated a number of times. Most recently in 2002 they published an updated report including urodynamic study findings. Appendix 1 reviews definitions from the report as they pertain to retention and bladder-emptying disorders.
“Lower urinary tract symptoms (LUTS)” now serves as a global term that applies to the variety of symptoms that may be representative of any bladder, urinary outlet, pelvic floor, endocrine, or neurologic abnormalities related to storage of urine and voiding. We are discovering that LUTS have a similar prevalence between men and women, and this prevalence increases in both groups with age. The EPIC study, a large multicenter survey study of 19,165 individuals 18 years or older in five countries using the 2002 ICS definitions for LUTS, found 64.3 % reported of at least one lower urinary tract symptom, with higher prevalence of storage symptoms in women compared to men (59.2 % vs. 51.3 %) and the opposite was true for voiding symptoms (men vs. women 25.7 % vs. 19.5 %). The overall presence of overactive bladder symptoms was 11.8 %, and the rate increased with age in both men and women.
Causes of Incomplete Bladder Emptying
Multiple potential sources contribute to incomplete bladder emptying (Fig. 13.2) and can be differentiated into dysfunction of the bladder or dysfunction of the bladder outlet.
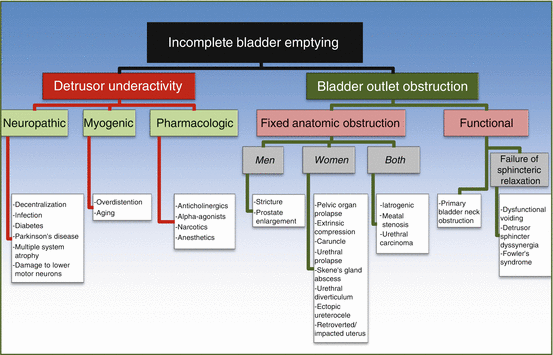

Fig. 13.2
Causes of incomplete bladder emptying
The ICS 2002 defines detrusor underactivity (DU) as “a contraction of reduced strength and/or duration, resulting in prolonged bladder emptying and/or a failure to achieve complete bladder emptying within a normal time span.” A movement toward the use of the term “detrusor underactivity (DU)” has been advocated to encompass this spectrum of diseases and to replace prior terminology used such as “impaired detrusor contractility,” “underactive bladder,” “detrusor areflexia,” “hypotonic bladder,” and “detrusor/bladder failure.” Detrusor underactivity is impressed upon because it places the focus of the condition more on the symptoms rather than the etiology, which, as we will discuss, are broad and quite variable, resulting from neuropathic, myogenic, or pharmacologic sources. Currently there is not an accepted definition for the clinical syndrome associated with DU. It has been suggested that the term underactive bladder (UAB) syndrome could be applied to the “clinical syndrome that accompanies detrusor underactivity.” The problem with this definition, as opposed to the overactive bladder syndrome (OAB), is that the symptoms associated with DU are variable and nonspecific. Osman et al. suggested that UAB could be defined as “reduced sensation of the need to void (the opposite of urgency) that may be associated with frequency and nocturia or reduced voiding frequency often with a feeling of incomplete bladder emptying and incontinence that may predominate at nighttime.” This definition has its obvious limitations.
Bladder outlet dysfunction, also coined as “bladder outlet obstruction (BOO),” may be a result of a fixed anatomic obstruction. Less obviously, it may be due to a functional abnormality in which no distinct structural abnormality is present, but rather the patient “functionally” has obstruction due to a neurological, myogenic, or psychological condition.
Detrusor Underactivity (DU)
Neuropathic Causes
Medical conditions including diabetic neuropathy and infections with HIV, herpes simplex virus, tertiary syphilis, or post-infectious polyneuritis causing Guillain-Barre are known to be causes of impaired detrusor function from neurological dysfunction. Parkinson’s disease is routinely found to cause detrusor overactivity but has been demonstrated to present with detrusor underactivity or acontractility. This has also been demonstrated in patients that suffer from upper motor neuron damage, particularly with hemorrhagic or cerebellar infarcts. Other spinal pathology primarily involving damage to lower motor neurons exiting from the lumbosacral vertebrae or sub-sacral lesions, such as from spinal cord injury, multiple sclerosis, trauma, or pelvic surgery, may result in detrusor underactivity or areflexia or poor urethral sphincter function. Consideration should also be given to cases of disk herniation, spinal stenosis, myelodysplasia, and cranial or spinal arteriovenous malformations as possible contributors.
Myogenic Causes
As discussed previously, it is postulated that ischemic detrusor denervation subsequently affects the entire myovesical plexus. The normal process of aging has also been associated with morphological changes to the detrusor muscle. Urodynamic studies have noted a loss of bladder contractility and voiding efficiency with increasing age. MRI imaging of the inula, the brain center that is responsible for processing visceral sensation, notes a diminished response to bladder filling in aging asymptomatic humans. In cases of chronic urinary retention, prolonged overdistention of the bladder results in changes to smooth muscle contractile function.
Pharmacologic Causes
Pharmacological influences on voiding are known to be very powerful. Despite their use for lower urinary tract symptoms, antimuscarinics may be overly effective in inhibiting detrusor function, resulting in incomplete emptying. Other classes of medications that impact bladder emptying include narcotics and alpha-agonists (phenylephrine, pseudoephedrine, clonidine).
Numerous studies and case reports have found asymptomatic or symptomatic postoperative urinary retention (PUR) following administration of regional or general anesthesia. Soon et al. reported a rate of urinary retention of 39.3 % and UTI in 24 % of patients who underwent surgery for hip fracture, with higher risk associated in those with longer hospitalization and higher 2-year mortality. This may have significant implications for those that require hardware for their repairs. Another study utilizing bedside ultrasound in the post-anesthesia care unit (PACU) in patients that underwent thoracic, vascular, abdominal, ENT, or orthopedic surgery found 44 % of the patients had bladder volumes of greater than 500 mL. If treated within one to two hours, however, volumes of 500–1000 mL were not harmful. Similar studies have found incidences of PUR in the PACU of 16 %. Factors predictive of PUR include age greater than 50, intraoperative fluids greater than 750 mL, bladder volume of greater than 270 mL on entry to PACU, male gender, obstructive preoperative symptoms, spinal/epidural anesthesia, prolonged postoperative analgesia, and anesthesia time greater than two hours.
Childbirth has also been associated with overdistention and possible overdistention injury. A recent study evaluating 8000 consecutive births found a 0.05 % incidence of prolonged voiding dysfunction. It has also been suggested that labor exceeding 700 min increases the risk for postpartum voiding dysfunction.
In cases in which the bladder has acute distention for a prolonged period of time, defined as “a bladder filling volume at the time of diagnosis of at least 120 % of a normal bladder capacity, which has lasted at least 24 h,” a new term has been introduced, “acute prolonged bladder overdistention” (ApBO), thought to be a consequence of spinal or epidural anesthesia, extensive pelvic or orthopedic surgery, or prolonged childbirth. It is often asymptomatic, leading to delayed treatment. ApBO differs from acute distention that is due to anatomic obstruction, which often initially is characterized by causing significant symptoms.
Bladder Outlet Obstruction (BOO)
Fixed Anatomic Obstruction
Differing etiologies exist between men and women that may serve as an anatomic source of obstruction. In men, benign prostatic enlargement (BPE) causing benign prostatic obstruction (BPO) is the most common and well studied. Urethral strictures, although may develop in women, are far more common in men. They may be affiliated with a history of sexually transmitted disease, balanitis xerotica obliterans, trauma, or an iatrogenic cause such as urethral catheterization or transurethral surgery. Obstructive female urethral pathology may be due to urethral prolapse, diverticulum, or caruncle. Other possible causes of obstruction may include pelvic organ prolapse, Skene’s gland abscess, a retroverted or impacted uterus during pregnancy, or extrinsic compression from, for example, a pelvic malignancy. Etiologies common to both genders include meatal stenosis, urethral carcinoma, or anti-incontinence surgery.
The latter is the most common cause of outlet obstruction in women. An estimated 2 % incidence of bladder outlet obstruction following surgery for stress urinary incontinence has been quoted in women based on data evaluating post-op urinary retention; however this may be an underestimation, as many women may have subclinical obstruction resulting in significant symptoms but no significant voiding dysfunction. Rates of voiding dysfunction, mainly obstruction, have been reported up to 33 % after autologous slings, 22 % after Burch, 20 % after retropubic, and 7 % and 4 % respectively for transvaginal needle suspension and tension-free vaginal tape. Suggested risk factors for postoperative obstruction include prior anti-incontinence surgery, concomitant pelvic organ prolapse, a lack of detrusor contraction on preoperative UDS, as well as a post-void residual greater than 100 mL and peak flow of less than 20 mL/s.
In men, a recent study of 117 patients that underwent artificial urinary sphincter (AUS) noted an increased incidence of postoperative urinary retention following transcorporal AUS placement. This was found in 32 % compared to 8 % of those that had a 4 cm or larger cuff placed in traditional fashion. Of those with a transcorporal AUS, 27 % versus 2 % of the traditional AUS underwent suprapubic tube placement. The overall urinary retention rate after AUS was 15 %. The mean duration of post-op catheterization was 6.5 days but lasted up to 6 weeks in some patients with suprapubic tube. They did report a lower rate of cuff erosion in those that had transcorporal cuff placement.
Functional Obstruction
Functional causes of outlet dysfunction are as a result of a poorly relaxing outlet rather than a fixed anatomic obstruction, caused either at the level of the bladder neck or the urethra and pelvic floor.
Primary bladder neck obstruction (PBNO) is when the bladder neck fails to open adequately during voiding. The two requirements for this diagnosis are lack of anatomic obstruction and lack of increased striated sphincter activity. It was initially thought to be an entity diagnosed most commonly in men ages 21–50, but more recently there has been increasing recognition of this condition in women as well. There is minimal data on its prevalence in children. It has been hypothesized to be due to either failure of degradation of mesenchymal elements, with incorporation of connective tissue and subsequent smooth muscle hypertrophy, or underlying neurologic pathology.
Failure of the sphincter or pelvic floor to relax can be a result of three conditions. One cause is thought to develop as a habitual contraction of the pelvic floor and/or urethral sphincter during micturition, perhaps in young children who have pelvic floor discomfort (from possibly abuse or constipation) or as a response to urinary urgency. These patients are often diagnosed as having dysfunctional voiding, which is characterized by variable contractions throughout a void that prevent normal emptying. It is now being recognized as also developing in adults that present with a myriad of voiding complaints, thought to be a compensatory response to detrusor overactivity by urethral sphincter contraction throughout voiding that then becomes habit. Often patients with dysfunctional voiding present with very bothersome storage and voiding symptoms. If a patient has an underlying neurological condition (e.g., spinal cord injury, multiple sclerosis) and have the same symptoms and study findings, the patient will be diagnosed with detrusor sphincter dyssynergia (DSD). This can further be subclassified into lack of coordination by the external sphincter (detrusor external sphincter dyssynergia; DESD), which may occur by supracervical lesions, or by the internal sphincter (detrusor internal sphincter dyssynergia; DISD), which occur with lesions that occur at or above the takeoff of the sympathetic efferents at T11–L2 (Fig. 13.1). Additionally, the presence of DESD and DISD can coexist together.
Fowler’s syndrome differs from dysfunctional voiding and DSD as patients are asymptomatic and are often in retention at time of diagnosis. It is a rare condition found in young women, typically postmenarche and in the second and third decades of life, with long-standing detrusor inhibition from a chronically non-relaxing external urethral sphincter. The original paper describing Fowler’s syndrome in 1988 made an association to polycystic ovaries because 14 of the 22 patients with abnormal EMG activity had the condition, which thereby postulated a hormonal cause. There is poor data to support this as of yet.
Clinical Practice: Diagnosing Incomplete Bladder Emptying
The proper workup and evaluation of the patient is important to identify patients at risk, offer appropriate therapies in a timely fashion, and avoid unnecessary tests and treatments. Regardless of the etiology of incomplete emptying, the subsequent symptoms that develop may be debilitating despite very minimal objective findings, or the opposite may occur in which upper tract dysfunction is identified in a patient who has minimal to no symptoms. The goals of evaluation should be to establish a diagnosis, to define the characteristics of the patient’s storage and emptying, and to identify patients at risk for long-term sequelae.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








