Modified lithotomy position
Digital rectal examination to assess level of tumor in relation to dentate line and fixation of tumor
Laparoscopic approach unless contraindicated
Complete mobilization of left colon
Identification of left and right ureters
Full splenic flexure mobilization
Isolation and high ligation of inferior mesenteric artery at aorta
Isolation and ligation of inferior mesenteric vein at level of duodenum
Total mesorectal excision
Critical assessment and preservation of bilateral ureters, hypogastric nerves, and nervi erigentes
Assessment of distal margin via palpation in pelvis and digital rectal examination
Digital vaginal examination to ensure adequate separation of the posterior vaginal and anterior rectal walls
Assess colon for length to perform restorative procedure
Plan specimen extraction and restorative procedure:
If adequate distal margin at 2 cm from dentate line:
Staple distal margin
Periumbilical specimen extraction
Staple proximal margin
Colonic J pouch construction if length permits
Straight circular anastomosis, coloplasty, or end-to-side anastomosis if J pouch not possible
Double stapled circular anastomosis
Air leak test with flexible sigmoidoscopy
If stapled anastomosis not possible due to distal margin less than 2 cm from dentate line:
Placement of transanal retractor and effacement of anus
Distal margin transection at or above dentate line using electrocautery
Mucosectomy or intersphincteric dissection
Transanal extraction of rectum and sigmoid colon
Transection of proximal margin
Colonic J pouch stapled construction if length permits
Hand-sewn coloanal anastomosis of J pouch or end-to-end configuration
Water test anastomosis via pelvic irrigation
Placement of pelvic drain
Construction of diverting loop ileostomy
Splenic flexure mobilization is routinely performed during all proctectomies. While non-randomized studies have shown that it is safe not to perform a splenic flexure mobilization in selected patients undergoing proctectomy, there are advantages to its routine use [25–27]. There are no differences in bowel perforation, anastomotic leak, post-operative morbidities, or oncologic outcomes if the splenic flexure is mobilized, though the average operative time is longer [25–27]. The average size of the extracted specimen is, however, longer in those patients with splenic flexure mobilization; therefore, mobilization facilitates the creation of a colonic reservoir [25]. Furthermore, it has been shown that patients who undergo splenic flexure mobilization are significantly more likely to receive a colonic reservoir than those who do not (49 % vs 22 %, p 0.039) [27]. A colonic J pouch should be routinely created as part of a coloanal anastomotic procedure whenever technically feasible. Routine ligation of the inferior mesenteric artery proximal to the left colic artery and the inferior mesenteric vein at the edge of the pancreas helps secure adequate length for a reconstruction. Moreover, routine splenic flexure mobilization and “high” ligation help ensure an optimal blood supply to a tension-free anastomosis. We evaluated 19 patients in whom a re-do low anterior resection or coloanal anastomosis was required to treat a prior colorectal anastomosis which had either leaked or strictured [28]. In 89 % of patients, a “high” ligation had not been performed and the splenic flexure had not been mobilized [28].
After a stapled anastomosis, regardless if the reconstruction is straight or a reservoir, air-leak testing should be performed. Routine intra-operative flexible sigmoidoscopy allows for the early detection of an anastomotic leak or small dehiscence as well as direct inspection of the staple line. Staple line visualization may identify bleeding or ischemic mucosa and may help reduce the risk of post-operative anastomotic complications [29]. Given that the risks of performing routine intra-operative flexible sigmoidoscopy are extremely small and the procedure does not add much operative time, routine performance is advocated to inspect the anastomosis [29, 30]. In the case of a distal hand-sewn coloanal anastomosis, flexible sigmoidoscopy is not possible to perform an air leak test. Instead, a “water leak test” is performed by using the reverse Trendelenburg position while the pelvis is filled with fluid. Additional sutures may be placed to reinforce the staple line if there is a water leak.
Hand-Sewn Versus Stapled Anastomosis
The majority of surgeons perform a stapled anastomosis whenever technically feasible. During the early days of stapled colorectal anastomoses, the results were conflicting. A randomized trial of the circular stapler in 1981 found that though the stapler carried risks of rectal tear and anastomotic defects, it allowed surgeons to “save as many as 12 % of rectums” where a pelvic anastomosis was required but a hand-sewn transanal anastomosis would have required additional rectal resection [31]. This finding is less relevant in the era of total mesorectal excision for rectal cancer. Other early studies showed that the stapled anastomosis was not associated with increased adverse outcomes, nor did it alter the risk of recurrence or survival [32–34]. However, a randomized trial of 118 patients requiring a low colorectal anastomosis concluded that the hand-sewn anastomosis should remain the standard of care [35]. In this study the early stapler did not save time, caused more complications, and was much more expensive [35]. Despite this study, work with staplers continued and results continued to improve [36, 37].
More recent studies have shown the benefits of the stapled anastomosis, leading to its widespread adoption in intestinal surgery, including restorative proctectomy. A stapled anastomosis now takes significantly less time than does a hand-sewn anastomosis; a mean of 50 min less time [38, 39]. Morbidity is similar between the techniques, although the stapled anastomosis has lower anastomotic leak and stricture rates [38, 40]. Disease-free survival, overall survival, and recurrence rates do not differ between the technique of anastomosis [39, 41]. The hand-sewn colorectal anastomosis has been shown to have equal or perhaps worse post-operative sphincter function, although there is an acknowledged bias in some of these studies where hand-sewn anastomoses are reserved for the very distal anastomoses where a stapled anastomosis would not be possible [38, 42, 43]. The most recent Cochrane review on the subject concluded that the stapled anastomosis should not be considered superior to the hand-sewn anastomosis in colorectal surgery, but found that there are few recent studies on the topic and are no longer relevant in the setting of elective surgery [44]. Operative time was not an included outcome in this review [44]. In summary, the stapled anastomosis is safe and takes less time than does a hand-sewn anastomosis. Where feasible, a stapled anastomosis should be performed, reserving the hand-sewn technique for very distal anastomoses where a restorative proctectomy would not otherwise be possible. Both techniques are very relevant and the surgeon treating distal rectal cancer must be capable of performing both of them.
For the stapled technique, a purse-string device is used to secure a continuous number 1 polypropylene suture at the proximal resection margin. Alternatively, a running number 1 polypropylene suture may be circumferentially sutured at the cut edge of the proximal resection margin of colon. Various diameter circular staplers are available. The 33 mm stapler is routinely used in the authors’ practice, with the 29 mm stapler being used as an alternative for small-caliber small bowel during some ileorectal anastomosis procedures. The distal spike of the stapler is brought out through the center of the staple line (or as close to it as possible). A Lone Star ™ rectractor is used to efface the anus if there is any difficulty passing the stapler up through the anal canal. Special attention must be given in female patients to ensure that the vagina is not entrapped in the stapled line prior to its firing. After the stapler is fired the proximal and distal anastomotic rings are inspected for complete circular integrity. These are separately labelled and sent to pathology, with the oncologic status of the anastomotic rings being of particular interest in a very distal rectal cancer and threatened distal margin. A clear correlation has been shown between the number of distal stapler cartridges used and anastomotic leak rate. For this reason, a distal purse string or a single stapler cartridge closure of the rectal stump is preferable to multiple stapler firings. For a hand-sewn anastomosis the Lone Star™ retractor is used to efface the anus. The anastomosis is oriented by placing 4 corner sutures of 2-0 Vicryl. Interrupted 2-0 Vicryl sutures are then placed circumferentially, about 3 mm apart. In both techniques, meticulous care must be taken to ensure the proximal colon is not twisted on its mesentery, the anastomosis is tension-free, and the 2 ends of bowel have good blood supply.
Colonic Reservoirs
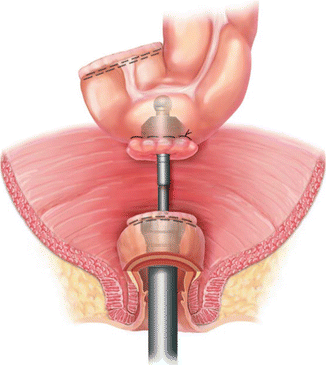
Whether stapled or hand-sewn, various configurations of the colon may be used for the colorectal or coloanal anastomosis. A straight or end-to-end anastomosis after rectal resection replaces the normally compliant rectum with a segment of sigmoid or descending colon (Figs. 15.1 and 15.2). Most patients have satisfactory bowel control and stool frequency with an end-to-end anastomosis [45]. However, there is a subset of patients who have very frequent bowel movements per day, especially in the setting of a very low anastomosis [46]. Other unsatisfactory symptoms after restorative proctectomy include incomplete evacuation, incontinence, clustering of bowel movements and urgency [47, 48]. Validated tools have been developed to quantify these symptoms, collectively known as low anterior resection syndrome (LARS) [47, 48]. Neoadjuvant radiotherapy, a coloanal anastomosis, and hand-sewn anastomosis are associated with poorer function [47]. Quality of life after restorative proctectomy is closely associated with the severity of LARS symptoms [49]. To mitigate these symptoms, various techniques have been studied in an attempt to re-create the reservoir function of the resected rectum. These are known as colonic reservoirs and include the colonic J pouch, transverse coloplasty, and end-to-side (or “Baker”) configurations.
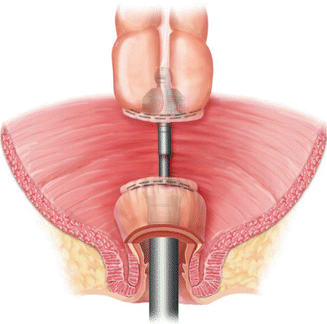

Fig. 15.2
Staple lines prepared for stapled end-to-end colorectal anastomosis (With permission Wexner and Fleshman [95])
Colonic J Pouch
The technique of creating a colonic J pouch is similar to that of an ileal J pouch, though the colonic J pouch should be much smaller. Healthy diverticular-disease-free descending colon is used for the pouch. The pouch should be assessed for adequate tension-free reach to the level of the distal rectal or anal resection margin. A 6–8 cm distance from the stapled proximal resection margin is measured and will form the efferent limb of the pouch. This limb is folded back onto the colon so that the anti-mesenteric borders are approximated. An enterotomy is then made at the apex of the pouch. A linear cutting stapler is introduced and fired along the anti-mesenteric borders to create the pouch. The staple line is irrigated and inspected for bleeding. Interrupted seromuscular sutures are placed at the level of the tip of the afferent limb for added support. For a stapled anastomosis, a purse string suture is then placed at the enterotomy and the anvil is secured. For a hand-sewn anastomosis, interrupted sutures are used to create the coloanal anastomosis. See Fig. 15.3 for an illustration of the anatomy and proper orientation of a colonic J pouch in the pelvis.
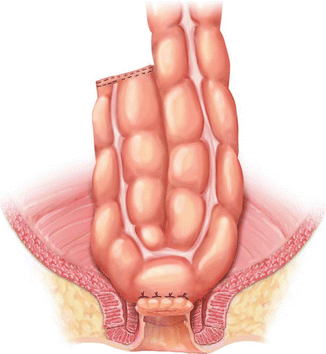

Fig. 15.3
Anterior view of a colonic J pouch in the pelvis (With permission Wexner and Fleshman [95])
The colonic J pouch was first described for use in coloanal anastomosis after proctectomy for rectal cancer. Early series found that with the colonic J pouch, the mean number of bowel movements was 1.1 per day, maximum tolerated volume was increased on anal manometry, and significantly more patients had 1–2 bowel movements per day when compared to patients with straight coloanal anastomoses [50, 51]. Although a randomized trial comparing colonic J pouch to straight coloanal anastomosis found that the capacity of the 2 reservoirs was not significantly different a 6 months, the colonic J pouch was associated with better functional results, including less stool frequency and less incontinence [52]. These benefits persist up to at least 1 year post-operatively [53, 54]. One study even found better functional outcomes 5 years after colonic J pouch reconstruction [55]. These superior outcomes included decreased frequency, urgency, and nocturnal bowel movements [55]. In addition the anal pressure gradient, mean pressures, compliance, and threshold volume are all better on anal manometry testing in patients with colonic J pouches, over straight anastomoses [55, 56]. These facts may explain why the quality of life in patients with colonic J pouches is better, especially up to 1–2 years after surgery [57]. In non-randomized trials, the anastomotic leak rate is also substantially lower following colonic J pouch [53, 58]. Randomized trials, a meta-analysis, and Cochrane review have all come to the same conclusion – that colonic J pouch is superior to straight anastomosis for at least 1 year after surgery [59–61]. The Association of Coloproctology of Great Britain and Ireland state that colonic J pouch formation should be considered in their position statement on the management of colorectal cancer [62]. Given these data, the colonic J pouch should be used whenever technically feasible for low colorectal and coloanal anastomoses in restorative proctectomy for rectal cancer.
Transverse Coloplasty
A coloplasty also creates a reservoir, but does not require as much redundant colon or as wide a pelvis as does a colonic J pouch. First, the colon must be assessed for length to see if a coloplasty will still allow a tension-free anastomosis in the pelvis. If so, the bowel is brought out through an incision or may also be brought out transanally if the distal resection margin is very low. Starting approximately 5 cm proximal to the proximal resection line, a 8–10 cm full thickness longitudinal colotomy is made along the anti-mesenteric border of the colon. Stay sutures are then used to reapproximate the colotomy transversely. Interrupted sutures are used to close the colotomy transversely, creating the colonic reservoir. A stapled or hand-sewn end-to-end anastomosis to the distal resection margin is then performed. Figure 15.4 illustrates a completed transverse coloplasty with a hand-sewn coloanal anastomosis.
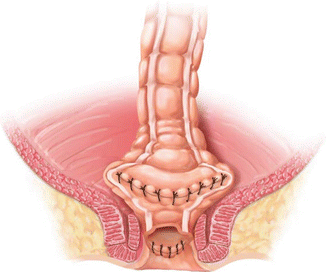

Fig. 15.4
Transverse coloplasty, shown with hand-sewn coloanal anastomosis (With permission Wexner and Fleshman [95])
Small randomized studies have compared the colonic J pouch to transverse coloplasty. When combined, the functional outcomes appear to be similar between the reservoirs [62–65]. One potential advantage of the transverse coloplasty over the colonic J pouch is that fewer patients have evacuatory difficulties or need for enemas [65, 66]. Most patients have up to 3 bowel movements per day [66]. It should be noted that one randomized trial found a trend toward more anastomotic leaks in the coloplasty group, but this was not statistically significant [65]. It is clear from many studies that both the colonic J pouch and coloplasty provide better functional results, quality of life, and superior manometry results over a straight anastomosis [67–69]. These same studies suggest that perhaps the best indication for a coloplasty is for patients in whom a colonic J pouch is not technically feasible; for example, in a narrow pelvis or a bulky mesentery [68, 69]. A study from the Cleveland Clinic found that since the technique of coloplasty was introduced, there were fewer failures in creating some form of colonic reservoir in patients requiring a coloanal anastomosis for rectal cancer [67]. Specifically, coloplasty was formed in patients in whom a colonic J pouch was not possible. However, a later multi-center randomized controlled trial which included the same institution concluded that in patients who could not have a colonic J pouch due to technical issues, coloplasty did not confer significant benefits over a straight anastomosis, perhaps because of their anatomical limits that prevented them from receiving a colonic J pouch as well [70]. Thus, the colonic J pouch should still be preferred over the coloplasty, though it is a reasonable reservoir option to consider when a pouch is not possible.
End-to-Side Anastomosis
An end-to-side, or “Baker” anastomosis, is typically secured to the distal resection line with a circular stapler, as shown in Fig. 15.5. The proximal resection margin is chosen in an area with good blood supply, free of diverticulae, and able to reach the distal resection margin without tension. There are two techniques used to introduce the anvil of the stapler. One approach is to sharply transect the specimen at the proximal margin. The anvil of the circular stapler is inserted through the open end of colon and the tip of the anvil is brought out through a small colotomy about 3 cm from the end of the colon on the anti-mesenteric border. The end of the colon is then closed with a linear stapler. A purse-string stitch is used to close the small colotomy around the post of the anvil. The alternative technique is to staple the transection margin of the colon. A larger, approximately 3 cm, colotomy is then made about 3 cm from the staple line on the anti-mesenteric border. A purse-string stich is placed and the anvil is inserted and secured in the same fashion as for an end-to-end anastomosis. The stapler is then fired from below under direct visualization, taking care of to keep the colon properly oriented.
The end-to-side anastomosis appears to confer many of the functional advantages of the colonic J pouch. Compared to a straight anastomosis, there are significantly fewer anastomotic leaks and similar overall anastomosis is safe, and easier and faster to create than the colonic J pouch [71, 72]. There are some subtle differences that make the colonic J pouch the preferred reconstructive option. The colonic J pouch has been shown to have better ability to evacuate in less than 15 min at 6 months post-operatively [73]. Maximal tolerable volume and volume of urgency is better in the early post-operative period in the colonic J pouch as measured by anal manomtery [74]. A 2008 Cochrane review of 4 randomized trials comparing colonic J pouch to side-to-end anastomosis did not demonstrate a functional difference between groups, but acknowledged that they were small trials and recommended larger randomized trials [62]. A more recent meta-analysis of 6 randomized trials found similar functional outcomes between the groups, but better early post-operative function in the colonic J pouch group [75]. For this reason, a colonic J pouch should be created if technically feasible. An end-to-side anastomosis is the second choice and a coloplasty is a possible third choice for reconstruction.
Colonic Rotation and Interposition
After full mobilization of the left colon and splenic flexure and high ligation of the inferior mesenteric vessels, some patients may still not have adequate colonic length to perform a restorative procedure. This problem may be attributed to prior colon resection, extent of the planned resection, or a simple variant of normal colon length. In order to achieve reconstruction in these patients, additional manoeuvers should be considered. A right colon to rectal anastomosis can be employed by fully mobilizing the right colon and rotating it into the pelvis, while maintaining ileocolic blood supply. This technique has been shown to have good results, with all patients having stoma closed, no anastomotic leak, and a mean of 3 bowel movements per day in a series of 48 patients [76]. Ileocecal rotation has also been shown to be successful after even more extensive resection of the colon [77]. After rotation of the ileocecal segment or right colon segment, the bowel may be brought through a window of the small bowel mesentery to facilitate reach into the pelvis. In addition to rotational procedures, interposition of pedicled segments of ileocecum or intervening colon have been described to allow for a reconstruction in the pelvis [78, 79]. While not commonly needed, rotational and interposition procedures are valuable tools to achieve a reconstruction after proctectomy, especially where a more extensive resection of the left colon is needed.
Fecal Diversion
A loop ileostomy should generally be created in the setting of a restorative proctectomy following neoadjuvant therapy for rectal cancer. It is created about 40 cm proximal to the ileocecal valve to facilitate subsequent closure. Meticulous care and inspection must be done to ensure proper identification of the afferent and efferent limbs during maturation. This is especially important during a laparoscopic procedure and the small intestine of each limb should be run to confirm correct orientation. The afferent limb should be everted during maturation to better facilitate pouching and minimize skin irritation. Stoma closure is often performed 12 weeks after surgery. Stoma closure should be delayed until all post-operative chemotherapy has been completed. Prior to stoma closure a water-soluble contrast enema and direct inspection of the anastomosis with sigmoidoscopy should be performed to rule out a sub-clinical anastomotic leak. Minor stricturing of the colorectal or coloanal anastomosis is usually easily dilated with the examining finger at the time of sigmoidoscopy and/or just prior to stoma closure if needed.
The need for a diverting stoma has been called into question, as stoma closure requires a second operation and hospital stay. However, numerous studies have shown that a defunctioning stoma is important after restorative proctectomy. Patients without a temporary ileostomy have significantly higher rates of anastomotic leak, peritonitis, and need for unplanned or urgent re-operations in the post-operative period [80–82]. Pooled analysis in a Cochrane review concluded that while a proximal stoma does not change post-operative or long term mortality after rectal cancer surgery, it does prevent anastomotic leaks and urgent re-operations [82]. Given that an ileostomy is safe and usually associated with only minor problems, such as dehydration, it should be recommended [82, 83]. It is vital to have enterostomal therapists and nurses who can provide education to patients on local stoma care, pouching, and most importantly fluid management and signs of dehydration. They are invaluable in reducing complications during the time a patient is diverted.
Complications and Functional Outcomes
Anastomotic leak is a risk in any intestinal surgery, but is especially significant after restorative proctectomy. A severe leak may result in the need for a permanent stoma in patients who return to the operating room and require intervention on the anastomosis. A diverting ileostomy significantly mitigates against the sequelae of a leak. Risk factors for anastomotic leak include malnutrition, smoking, obesity, and chronic disease [84]. A low anastomosis, prolonged operative time, and spill of bowel contents during the procedure are also risks that are common in restorative proctectomy for rectal cancer [84]. A quality improvement study of colorectal resections found that anastomosis less than 10 cm from the anal verge was an independent predictor of anastomotic leak [85]. Long term consequences after anastomotic leaks persist up to 3 years. At 1 year following restorative proctectomy, patients who had a post-operative leak have been shown to have more frequent bowel movements and worse bowel control [86]. At both 1 and 3 years after restorative proctectomy, these same patients also have worse mental component scores on the Short-Form 36 questionnaire [86]. A defunctioning stoma and close observation in the early post-operative period are keys to preventing, identifying, and minimizing the consequences of anastomotic leak.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree