Fig. 3.1
True resistant hypertension (blue bar) and pseudoresistant hypertension (green bar) in CKD. (Adapted from Nicola et al. [31]. With permission from Elsevier)
Observational studies have documented a high prevalence of RH in patients with CKD [5–8]. Despite the use of multiple antihypertensive drugs, hypertension remains above goal in patients with diabetic and nondiabetic CKD [9, 10].
Etiological Factors for RH in Patients with CKD
The pathogenetic mechanisms for RH in patients with CKD and ESRD are complex and multifactorial, with an interdigitating basis . The classical explanation for RH in CKD is an adverse interplay between intravascular volume and the renin–angiotensin–aldosterone system (RAAS). Hence, the traditional therapy for hypertension in CKD patients has been based on reducing the volume and or using RAAS blockade. However, it has become evident that despite volume control and adequate RAAS blockade, BP remains out of control in most patients with CKD. Thus, additional etiopathogenetic factors may be operative in sustaining elevating BP levels in patients with CKD. Among these alternate pathways of persistent RH in CKD are—inappropriate activation of sympathetic nervous system (SNS) , enhanced production of endothelin (a potent vasoconstrictor), decreased availability of nitric oxide (NO) , blunted endothelial function, obstructive sleep apnea (OSA) , and structural changes in the arterial tree. Furthermore, therapies for underlying renal disease—such as steroids, cyclosporine, calcium/vitamin D, erythropoietin, and nonsteroidal anti-inflammatory drugs (NSAIDs) can increase the BP in patients with CKD (Table 3.1).
Table 3.1
Resistant hypertension: factors
Noncompliance to therapy |
Excessive salt intake |
Increasing body weight |
White coat hypertension |
Drug–drug interactions |
Excessive alcohol use |
Drugs which cause hypertension—steroids, nasaids, erythropoietin, cyclosporine, certain herbal preparations, etc. |
Obesity and metabolic syndrome may also participate in the pathogenesis of hypertension in CKD.
An important pathophysiological observation in patients with CKD is excess sodium and water retention (due to ↓GFR). The consequent expansion of extracellular volume (ECV) makes the BP control difficult and is the cause of resistance to antihypertensive drugs even at high doses. ECV expansion is inversely related to GFR, thus creating an unfavorable connective loop. Even in the absence of visible edema, ECV expansion is a potent factor in the development of RH. Salt sensitivity and volume retention are twin interactive features of sustained hypertension in patients with CKD [11, 12]. The presence of nocturnal hypertension (nondipping status) is also indicative of ECV expansion in patients with CKD.
It is well known that inappropriate activation of RAAS despite volume excess contributes to sustained hypertension in patients with CKD. From a physiological point, ECV expansion should inhibit the activity of RAAS. However, in patients with CKD such an inverse relationship between volume and RAAS is lost. Any level of RAAS in the face of volume expansion therefore raises the BP level. On this basis, RAAS blockers are widely used as antihypertensive strategy in patients with CKD. BP response to RAAS blockade is indirectly reflective of the role played by RAAS in the pathogenesis of hypertension in patients with CKD.
Increased SNS Activity
Augmented activity of SNS has been demonstrated in CKD. Kidney is a sensor of systemic circulation and is endowed with intrinsic SNS functional activity—both efferent and afferent nerves. The kidney is not only a target of SNS but also a dynamic reservoir of SNS functions. Activation of chemoreceptors in the kidney (by ischemic or uremic toxins) precipitates nerve traffic to and from the central nervous system. Chronic stimulation of the renal (afferent) nerves leads to SNS activation with resultant hypertension (Fig. 3.2). The synthesis and turnover rate of norepinephrine from the hypothalamic region are enhanced in experimentally induced renal dysfunction [13]. Muscle sympathetic nerve activity (MSNA) is excessive in patients with CKD compared to normal. It is possible that excessive SNS activity in CKD may also be mediated to some extent by the RAAS. Reduced baroreceptor function and increased levels of leptin also contribute to SNS activation in CKD.
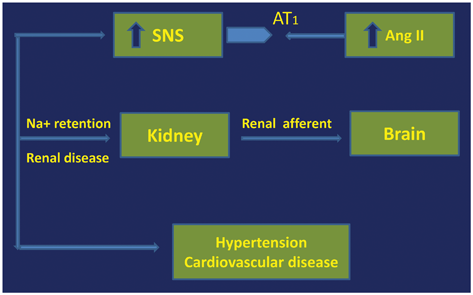
Fig. 3.2
Neurogenic factors in renal hypertension
Endothelin, CKD, and Hypertension
Endothelin-1(ET-1) a peptide derived from endothelial cells is a powerful vasoconstrictor. ET-1 exerts a number of biological effects including vasomotor tone, cell growth, (renal) sodium, and water retention . Thus, excessive activity of ET-1 impacts systemic vascular resistance (SVR) and the BP level. Studies have demonstrated that the ET system is important in the pathophysiology of CKD-associated hypertension [14–16]. A number of intrarenal mechanisms (cytokines, protein load, nephron loss, decreased GFR) may stimulate ET-1 production which can cause hypertension and further progression of CKD. ET-1 levels correlate with the level of renal function and proteinuria in diabetic nephropathy [17]. It is possible that the deleterious consequences of ET-1 may also be mediated via SNS and RAAS in the pathogenesis of advancing hypertension associated with CKD .
Obstructive Sleep Apnea
OSA is independently linked to hypertension and related CVD [18–21] . A number of pathways in OSA principally mediated by hypoxia cause vasoconstriction and hypertension. It has been reported that OSA is more prevalent in patients with CKD compared to the controls [22–25]. OSA has been correlated with GFR and proteinuria in patients with CKD but whether OSA is an independent risk factor for CKD is not established.
Circadian Variability of BP
It has been suggested that in patients with CKD, normal circadian variability of BP is lost . Normally, BP levels are highest in the early morning gradually decreasing during the course of the day and reach a low level during sleep (“dippers”). The so-called nondippers do not display such BP variability and their nocturnal BP does not drop. In patients with CKD and ESRD, the prevalence of “nondippers” status is high [26–29]. The occurrence of nondipping and therefore nocturnal hypertension in patients with CKD is a factor in the genesis of RH in patients with worsening renal function. Nocturnal hypertension is associated with significant target organ damage (TOD) and excessive cardiovascular events in patients with CKD .
Oxidative Stress
Oxidative stress, a state of imbalance between production and breakdown of reactive oxygen species (ROS), is a marker of vascular function. A high production of ROS causes vascular dysfunction. Excessive production of ROS causes intense vasoconstriction and hypertension. Thus, ROS can cause severe hypertension directly or by depleting nitric oxide (NO) . Oxidative stress has been proposed as a possible link between CKD and severe hypertension [30]. Renal damage is associated with oxidative stress even in the early stages of CKD. The oxidative stress not only causes vigorous vasoconstriction but also accelerates kidney injury. Oxidative stress, a potent factor in the development of CVD also plays a role in the occurrence of vascular disease and pathogenesis of hypertension in patients with CKD.
RH in Patients with CKD: Significance and Prognosis
Hypertension in patients with CKD often requires utilization of multiple antihypertensive drugs in maximum doses . A substantial number of patients, however, remain “resistant” to optimal combination of potent antihypertensive drugs. Typically, RH is defined as BP that remains above therapeutic goals despite the concurrent use of three antihypertensive drugs or requirement of four or more classes of drugs to achieve the goal BP level. The so-called treatment RH is common and severe in patients with CKD. Treatment RH in patients with CKD predisposes to adverse cardiovascular outcomes and premature death.
RH in patients with CKD is associated with lower estimated GFR (eGFR) and proteinuria. In addition to aggressive therapy for hypertension, it is important to identify the factors for the decline of renal function in patients with CKD. Close follow-up is recommended based upon the level of BP, GFR, and serum potassium. RH in CKD is often volume-dependent; hence, effective diuretic treatment is of critical value. Selected causes of RH include :
Excessive sodium intake
Fluid retention
Inadequate diuretic dosage
Erroneous choice of a diuretic
Inappropriate combination of antihypertensive drugs
Drug–drug interactions
Drug-induced BP elevation
Obesity
Sleep apnea
Insulin resistance
After excluding aggravating/causative factors, intense BP monitoring and aggressive therapy should be implemented. Volume control is of major benefit in patients with CKD who have RH. With the understanding that RH is a risk factor for rapid decline in renal and cardiovascular functions, BP control is of immense importance . Persistent hypertension in patients with CKD is due to an interplay of etiological factors such as volume overload, increased activity of the RAAS and of SNS. ECV expansion in patients with CKD is directly related to the degree of renal failure; thus, tight control of volume is advocated along with blockade of the RAAS and SNS to improve the BP levels in patients with CKD. Renal denervation (RDN) therapy may be indicated for some patients with CKD who have persistent, severe, complicated, or RH.
RH increases the morbidity and mortality in patients with CKD. Uncontrolled hypertension causes further renal damage, which in turn causes rapid deterioration in BP control. It is a vicious cycle. While the office and clinic BP measurements are routinely used to classify and treat hypertension in patients with CKD, ambulatory BP monitoring (ABPM) is the best method to identify true RH in clinical practice. Various cardiorenal events in patients with RH can be correlated to the BP status as determined by ABPM [31, 32]. Greater application of ABPM in patients with CKD will likely identify those patients who need most rigorous antihypertensive therapy .
It should be clearly understood that RH in patients with CKD predisposes to serious adverse cardiorenal outcomes [33, 34]. RH is a powerful harbinger of adverse prognosis in patients with CKD [35, 36]. RH increases the risk of renal death. RH in contrast to treatable hypertension predicts CVD in patients with CKD; on the other hand, patients with pseudoresistance have favorable prognosis. One can surmise then that persistence of hypertension despite optimal antihypertensive treatment identifies individuals with severe and advancing vascular disease and “fixed” structural vascular abnormalities. Conditions associated with RH in patients with CKD include—left ventricular hypertrophy, diabetes, proteinuria, and high salt intake. Not surprisingly, these concomitant disorders are also accompanied by increased pulse wave velocity, endothelial dysfunction, and arterial stiffness. While a low GFR is a recognized risk factor for premature mortality in patients with CKD, proteinuria is a better marker of CVD .
Principles of Treating RH in Patients with CKD
The complex pathophysiological mechanisms of RH in patients with CKD dictate application of multiple therapeutic strategies to control hypertension in this high-risk population . In addition to hypertension, there are other factors which contribute to the progression of CKD to ESRD; these include (but are not limited to) hyperlipidemia, obesity, diabetes, tobacco use, and OSA . Hence, all of these predisposing factors should also be addressed in the management of patients with CKD. One of the cornerstones of BP reduction in patients with CKD is restriction of sodium intake to prevent ECV expansion. Patients with CKD are particularly prone to the negative consequences of high salt intake. Excessive salt intake may also trigger other markers of CKD such as oxidative stress, endothelial damage, proteinuria, and vascular inflammation. There is evidence to suggest that salt restriction is beneficial to treat RH in patients with CKD. Unless there are contraindications, salt restriction is recommended as an initial measure to manage RH. Most guidelines recommend an upper limit of 100 mmol of sodium per day (= 2300 mg or 6 g sodium chloride). Dietary education and counseling are critical measures to ensure that the CKD patients understand and comply with salt restriction .
The definition of RH includes optimal use of a diuretic. However, in patients with CKD, the selection and dosage of a diuretic are important to control the volume status. The choice of a diuretic and its dosage are dictated by the level of kidney function and stage of CKD. Patients with mild renal dysfunction (GFR > 40 ml/min) may respond to thiazide diuretics. With advancing CKD, loop diuretics with proper dosing and titration are recommended [37]. The dosage of loop diuretics has to be adjusted to correct sodium retention and to obtain desired weight reduction. Adequate use of loop diuretics is strongly recommended to treat RH in CKD patients unless there are contraindications. The uncommon phenomenon of “diuretic resistance” can be overcome by judicious addition of drugs like metolazone, which permit additional inhibition of sodium reabsorption in the distal tubule. Studies have also shown that aldosterone antagonists such as spironolactone may be a useful addition to treat RH in CKD patients [38]. Despite the efficacy of aldosterone antagonists in RH in CKD patients, these drugs should be used with great caution and under close surveillance due to the risk of hyperkalemia .
RAAS Blockers
RAAS blockers like angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are frequently indicated to treat RH in CKD patients. As a rule, RAAS blockers are used in combination with other classes of antihypertensive drugs to treat hypertension in general, and in CKD patients in particular. Despite their pharmacological benefits and protection against TOD , RAAS blockers have not been shown to reduce all-cause mortality in patients with CKD. ACEIs have been shown to exert significant anti-proteinuric and antihypertensive effects in patients with CKD [39]. ACEIs lower the intraglomerular pressure by dilating the efferent arterioles. The reduction in intraglomerular pressure protects the kidney. ARBs like ACEIs exert beneficial antihypertensive, anti-proteinuric, and renal protective properties. ARBs are well tolerated and effective in managing hypertension in patients with CKD. The combination of ACEIs and ARBs has not been shown to offer any advantages in treating hypertension; the combination may show additive effects on proteinuria reduction but no additional outcome benefits. Dual RAAS blockade is not helpful for BP control or target organ protection. Trials with direct renin inhibitors have not yielded any advantages or favorable outcomes and thus are not recommended routinely. The use of RAAS blockers to treat RH in CKD patients while necessary requires close vigilance of renal function and potassium level. Despite the advocacy of RAAS blockade in controlling hypertension, these drugs should be used with considerable caution in patients with worsening renal function (e.g., serum creatinine > 2.5 mg/dl and K + level > 5.5 meq/l).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






