Fig. 7.1
Practical recommendations for the use of renal denervation in clinical practice according to the European Society of Hypertension. (Reprinted from Schmieder et al. [23]. With permission from Wolters Kluwer Health)
To maximise safety, the committees recommend that patients who have previously undergone renal artery intervention, have evidence of renal artery atherosclerosis or impaired kidney function (estimated glomerular filtration rate (eGFR) < 45 ml/min per 1.73 m2) be exempt. Anatomical contraindications including multiple renal arteries, one kidney or a main renal artery of < 4 mm diameter or length < 20 mm should also exclude a patient from undergoing the procedure .
Clinical Trial Data on Catheter-Based Renal Sympathetic Denervation
The long-term safety and efficacy of catheter-based renal denervation to control BP has, to date, been evidenced by the Symplicity Clinical Trial Program. In 2009, the first proof-of-principle trial (Symplicity HTN-1) [20] was undertaken in 45 patients with resistant hypertension with an inclusion systolic BP threshold of > 160 mmHg (> 150 mmHg for patients with diabetes). Office BP was significantly reduced by − 14/− 10 mmHg (SBP/DBP) at 1 month after renal denervation with more pronounced reductions of − 22/− 11 mmHg and − 27/− 17 mmHg observed at 6 and 12 months, respectively. Renal sympathetic nerve activity as assessed by renal noradrenaline spillover was reduced on average by 47 %, confirming the impact of the denervation procedure on renal sympathetic nerve activity. Central sympathetic outflow, as assessed by microneurography, was also reduced in treated patients [25]. Importantly, no major procedure-related adverse events were reported.
As observed in a larger, extended Symplicity HTN-1 cohort (n = 153), the treatment effect on BP was sustained at 24 months [26] and, most recently, at 36 months [27], suggesting the absence of functionally relevant reinnervation of sympathetic nerves (Fig. 7.2). Four complications in the cohort included one renal artery dissection and three femoral artery pseudo-aneurysms. In 81 patients with magnetic resonance angiography, CT or duplex renal artery assessment post denervation, no stenosis was identified at sites where denervation was performed.
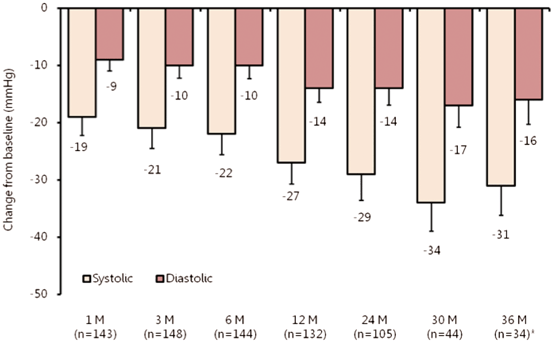
Fig. 7.2
Mean changes in office-based BP after renal denervation with up to 36-month follow-up in the extended Symplicity HTN-1 cohort (n = 153). Error bars represent 95 % confidence intervals (CIs). Compared to baseline, significant differences in office-based BP were observed for patients at all reported time points during the 36-month follow-up period (P< 0.01). Asterisk denotes number of patients with data available at time of data-lock
Encouraging results from the initial Symplicity HTN-1 trial led to the conduct of Symplicity HTN-2, a multicentre, prospective, randomized controlled trial involving 106 resistant hypertensive patients from 24 centres across Europe, Australia and New Zealand [28]. Of the 49 patients who immediately underwent renal denervation , mean office BP at 6 months significantly decreased by − 32/− 12 mmHg with no change in BP reported in the control group (n = 51) assigned to standard pharmacological therapy (Fig. 7.3).
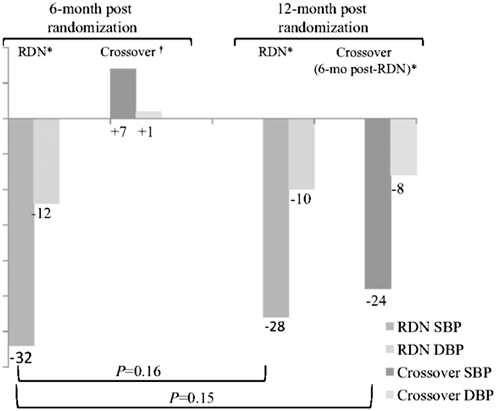
Fig. 7.3
Mean change in office-based BP after renal denervation at 6 and 12 months in the Simplicity HTN-2 trial. Both the initial renal denervation group and the crossover group denervated at 6 months after randomization experienced significant drops in systolic and diastolic BP. RDN denotes patient group immediately assigned to renal denervation at baseline, crossover denotes patient group who underwent renal denervation after randomization, DBP diastolic blood pressure, SBP systolic blood pressure. Asterisks denote P < 0.001 for SBP and DBP change after renal denervation; the dagger symbol denotes P = 0.026 for SBP change from baseline and P = 0.066 for DBP change from baseline for the crossover group before denervation at 6 months (Reprinted from Esler et al. 2012. With permission from Wolters Kluwer Health)
A subset of patients in the Symplicity HTN-2 trial (20 in the RDN group and 25 in the control group) underwent ABPM at 6 months, and the mean reduction in BP was 11/7 mmHg in patients with RDN, whereas there was no significant change in controls. Not surprisingly, the reduction in ABPM was less pronounced than the reduction in office BP. Other trials have confirmed that RDN causes greater reductions in office BP than ambulatory BP; however, the magnitude of the difference between office BP and ambulatory BP changes appears to be somewhat more pronounced than that observed in BP-lowering trials using pharmacological approaches.
Renal artery imaging at follow-up (n = 43) confirmed the safety of the procedure with no reported incidence of renal artery stenosis or aneurismal deformation.
Recently, 12-month follow-up data from 47 patients in the Symplicity HTN-2 trial were published [29]; also included were 6-month post-denervation data for 35 control patients who, per-protocol, elected to undergo to the procedure after randomization. Compared to at baseline, there was no additional reduction in patient’s office BP at 12 months compared to at 6 months (P = 0.16; Fig. 7.3).
The magnitude of SBP reduction at 12 months was, however, consistent with that observed in the first Symplicity HTN-1 trial (− 28 vs. − 27 mmHg). Mean change in office BP at 6 months was also shown to be comparable between patients assigned to immediate renal denervation and those who underwent the procedure after randomization (P = 0.15).
In terms of safety, only two peri-procedural events were reported. One control patient experienced a femoral artery pseudoaneurysm prior to renal denervation that was resolved without further sequelae. A second control patient was hospitalised following renal denervation for a hypotensive episode that was managed with a reduction in their antihypertensive medication.
In both cohorts, renal denervation preserved kidney function as evidenced by nonsignificant changes in eGFR, serum creatinine and Cystatin C at 6 and 12 months. The observation supports a recent study of 88 patients with resistant hypertension who had a preserved eGFR 6 months post renal denervation [30].
Symplicity HTN-3 [31] is the largest clinical trial on the safety and efficacy of renal denervation thus far, comprising a total of 535 patients with resistant hypertension randomized in a 2:1 ratio to receive renal denervation or a sham procedure. As the US pivotal trial seeking FDA approval, it was rigorously designed taking into account limitations that have been identified with Symplicity HTN-1 and 2. As such, patients had to have a systolic office BP of ≥ 160 mmHg while being on full doses of 3 or more antihypertensive drugs, including a diuretic. Ambulatory BP monitoring was mandatory and the 24-hour average BP had to be ≥ 135 mmHg to be included. Randomization occurred in the catheter lab after confirmation of suitable anatomy by renal angiogram. Patients were followed up by physicians blinded to the patient’s randomization status. The primary safety end point was a composite of major adverse events at 1 month. The primary efficacy end point of the study was the difference in the reduction of systolic office BP between the renal denervation and the sham group with a 5-mmHg superiority margin.
The primary safety end point was met with no difference in the major adverse event rate between the two groups (1.4 % in the renal denervation group vs. 0.6 % in the sham-procedure group). However, while there was a significant reduction in systolic office BP of − 14.1 ± 23.9 mmHg (P < 0.001) at 6-month follow-up in the renal denervation group, the difference in the change of BP with a superiority margin of 5 % (− 2.39 mmHg; 95 % CI, − 6.89–2.12; P = 0.26) was not statistically significant from that seen in the sham-procedure group (− 11.7 ± 25.9 mmHg; P < 0.001; Fig. 7.4), therefore the primary end point was not met. Similarly, the difference in the reduction of ABPM between the two groups, a secondary efficacy end point was also not met (RDN, − 6.75 ± 15.11 mmHg vs. Sham, − 4.79 ± 17.25 mmHg; difference in changes, − 1.96 (95 % CI, − 4.97–1.06); P = 0.98).
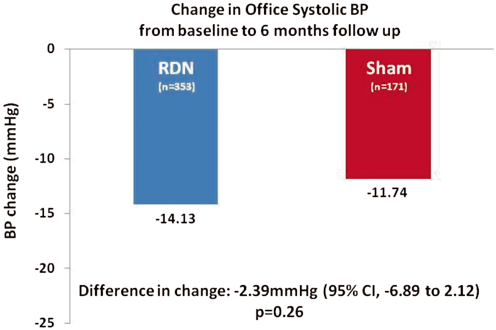
Fig. 7.4
Mean change in office systolic BP from baseline to 6-month follow-up in the Symplicity HTN-3 trial. A significant change from baseline to 6 months in office systolic blood pressure was observed in both study groups. The between-group difference (the primary efficacy end point) did not meet a test of superiority with a margin of 5 mmHg. The I bars indicate standard deviations
The results from this trial were in stark contrast to the results of all other studies using different denervation systems, most of which were uncontrolled studies, and were considered by many as a substantial setback for renal denervation as a therapeutic approach to resistant hypertension. Indeed, the trial was excellently designed and the results highlighted the relevance of a sham control in device-based studies. However, several aspects relating primarily to the conduct of the study have been criticized and discussed as potential contributors to the failure of the trial to meet its efficacy end points. These include (i) inexperience of operators (88 centres participated in the trial with 111 operators performing RDN in 364 patients without previous experience in RDN; (ii) a substantial number of patients (~ 40 % in each group) had medication changes within the first 6 months after the RDN or sham procedure; (iii) no measures of drug adherence were obtained and (iv) no evidence of the degree of renal denervation achieved during the trial could be obtained. Furthermore, in contrast to previous studies, ~ 25 % of participants in Symplicity HTN-3 were of African American background with subgroup analysis indicating a potential difference in the BP response in this patient group. Future and more detailed analyses of Symplicity HTN-3 will have to determine whether or not these factors may have influenced the results of Symplicity HTN-3. Irrespective of these findings, more research in form of adequately designed studies will be required to ultimately determine the role of RDN in the treatment of resistant hypertension .
Possible Utility of Renal Sympathetic Ablation Beyond Resistant Hypertension
Preliminary studies suggest that catheter-based renal denervation may have therapeutic benefits, beyond BP control, in patients at high risk of cardiovascular events.
Excessive sympathetic activation is a hallmark of both chronic kidney disease (CKD) and end-stage renal disease (ESRD) . In the vast majority of patients, chronic elevation in BP from sympathetic overdrive potentiates the progressive deterioration of renal function and leads to increased risk for serious cardiovascular events [32, 33]. Two pilot trials were recently undertaken to assess the feasibility and short-term safety of renal denervation in patients with CKD [34] and ESRD [35]. To date, only patients with normal kidney function (eGFR > 45 mL/min per 1.73 m2) have been assessed in large clinical cohorts [28, 29].
In 15 patients with resistant hypertension and stage 3–4 CKD (mean creatinine-based eGFR 31.2 [SD:8.9] mL/min per 1.73 m2), renal denervation was shown to safely reduce seated office and night time BP (as measured by 24-h ABPM) by − 32/− 15 mmHg and − 10/− 3 mmHg, respectively, at 6 months. Importantly, angiographic evaluation after the procedure revealed no compromise of treated arteries or disturbances in renal blood flow, electrolytes and eGFR. Improvements in peripheral arterial stiffness were also observed at 3 months.
For nine patients with ESRD and uncontrolled BP, a sustained reduction in office SBP of − 18, − 16 and − 28 mmHg at 3, 6 and 12 months, respectively, was observed following renal denervation . Patients also demonstrated a reduction in both sympathetic outflow, as measured by muscle sympathetic nerve activity , and in renal and whole body noradrenaline release at 3 months (n = 2). Anatomical limitations prevented three patients from undergoing renal denervation. Two patients also developed peri-operative femoral pseudo-aneurysms that resolved without further sequelae. Larger clinical trials are now warranted to substantiate these initial findings and determine whether renal denervation may represent a useful therapeutic approach in patients with impaired kidney function.
Chronic activation of the central sympathetic nervous system has also been implicated in the initiation and progression of several cardiovascular conditions that increase morbidity and mortality, including LVH, cardiac arrhythmias and chronic heart failure, at times in the absence of elevated BP [36]. Brandt et al. investigated the impact of renal denervation on LVH in 46 patients with resistant hypertension [37] and found the procedure significantly reduced LV mass, increased LV ejection fraction and improved diastolic function at 1 and 6 months.
A recent pilot study [38] evaluated the safety of renal denervation in seven normotensive patients with chronic systolic heart failure. At 6 months, all patients showed an improvement in their functional capacity (as assessed by a 6-min walk test) and overall quality of life. Of note, a recent study confirmed a beneficial effect on health-related quality of life after renal denervation [39]. Importantly, no procedural complications or symptomatic adverse effects were reported. Renal haemodynamics and function were also preserved.
Atrial fibrillation (AF) is associated with a sustained elevation in BP and represents the most common clinically significant cardiac arrhythmia. Usual treatment for AF includes catheter ablation to disconnect the pulmonary veins from the left atrium (known as pulmonary vein isolation; PVI). In patients with resistant hypertension , a combined therapy of PVI and renal denervation (n = 13) significantly lowered office BP and had a salutary effect on AF patterns compared to PVI alone (n = 14) [40]. At 12 months, 69 % of patients assigned to PVI with renal denervation were AF-free compared to 29 % assigned to PVI only. Patients on combined therapy also demonstrated a significant and sustained BP reduction of − 25/− 10 mmHg and reduction in LV mass of approximately 10 % at follow-up.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






