Reference
Published year
Animal
Model
Treatment
Study duration
Blood pressure
1
2015
Wistar rat
NO synthase inhibition
DNx
10 weeks
↓
2
2014
Female SHR
UNx
DNx
3 weeks
↓
3
2013
SHRSP
8 % NaCl diet
DNx
2–4 weeks
↓
4
2013
129S1/SvImJ mouse
UUO
DNx
10 days
N/A
5
2013
SHR/cp
Metabolic syndrome
DNx
19 weeks
↓
6
2012
Sprague-Dawley rat
UNx + aortic regurgitation
DNx
24 weeks
→
7
2011
SHR
DM induced by STZ
DNx
45 days
→
8
2010
Dahl salt-sensitive rat
UNx + 8 % NaCl diet
DNx
6 weeks
→
9
2008
Sprague-Dawley rat
Anti-Thy-1.1 nephritis
DNx
1 week
N/A
10
2008
SPRD-Cy/+ rat
ADPKD
DNx
4 weeks
↓
11
2006
Sprague-Dawley rat
Chronic AII infusion
DNx
5 weeks
↓
12
2005
Sprague-Dawley rat
UNx + DOCA-salt
DNx
47 days
↓
13
2004
Sprague-Dawley rat
DM induced by STZ
DNx
2 weeks
→
14
2002
Sprague-Dawley rat
UNx + cigarette smoke
DNx
12 weeks
→
15
2000
Sprague-Dawley rat
UNx + cyclosporine A
DNx
3 weeks
→
16
1995
Sprague-Dawley rat
5/6 nephrectomy
DR
6 weeks
↓
17
1995
Sprague-Dawley rat
5/6 nephrectomy
DR
6 weeks
↓
18
1994
Sprague-Dawley rat
NO synthase inhibition
DNx
4 weeks
↓
2 Materials
2.1 Animals and Induction of Anesthesia
1.
2.
Anesthetic: inhalational anesthetic (e.g., sevoflurane), 10 % sodium pentobarbital in saline (v/v).
3.
Warming plate (heated pad) to keep animal warm during surgery.
2.2 Approach to Renal Sympathetic Nerve
1.
Stereoscopic microscope. We use a Wild M10 (Leica, Wetzlar, Germany), but any other stereoscopic microscopes are acceptable.
2.
Surgical instrument and additional materials: fine blunt-tipped microsurgical tweezers, scissors, two microsurgery hooks (consisting of a clip and an elastic band), cotton swabs, and gauze dressings (Fig. 1).
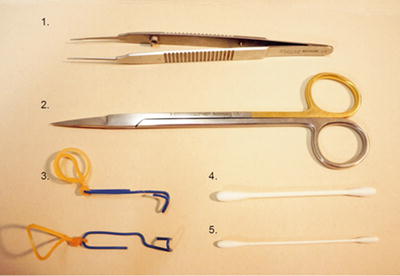

Fig. 1
Surgical instrument and additional materials for renal denervation. (1) Fine blunt-tipped microsurgical tweezers, (2) scissors, (3) two microsurgery hooks (consisting of a clip and elastic band), (4) cotton swabs, (5) and fine-tipped cotton swabs
2.3 Renal Sympathetic Denervation
1.
2.
Denervation solution: phenol in ethanol (10 % v/v). Phenol and absolute ethanol should be high purity grade chemicals. Melt phenol with hot water, pipette it, and add in ethanol quickly to avoid re-solidification in a pipette tip (see Note 2 ).
2.4 Verification of Renal Sympathetic Denervation (a Measurement of Kidney Norepinephrine Content)
1.
Anesthetic: inhalational anesthetic and 10 % v/v of sodium pentobarbital in saline.
2.
Surgical instrument and additional materials: tweezers, scissors, cotton swabs, and 21-gauge butterfly needles (Terumo, Tokyo, Japan).
3.
50 mL plastic syringe.
4.




Ice-cold phosphate-buffered saline (PBS; pH 7.4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







