Fig. 3.1
Common pathogenesis of CKD after AKI. Initial loss of nephrons and interstitial scarring leads to hyperfiltration and proteinuria. Hyperfiltration may maintain total GFR, but at the expense of progressive renal injury, which in turn accelerates these processes. Many risk factors may accelerate the progression of CKD, in many cases with positive feedback as CKD in turn is a significantly associated with development of cardiovascular disease, hypertension, systemic inflammation and risk of recurrent AKI. AKI acute kidney injury, CKD chronic kidney disease, RAAS renal/angiotensin aldosterone system
Evidence for the above mechanisms can be found in the outcomes of animal models of AKI. In rats after renal ischaemia/reperfusion (I/R) injury, creatinine levels return to the normal range after 4 weeks, despite a persistent GFR reduction of ~50 % [20]. Furthermore, a high salt diet causes hypertension and proteinuria in animals exposed to renal I/R, but not in sham operated controls [20]. Similarly a high salt diet reduces renal clearances and increases interstitial inflammation specifically in kidneys subjected to I/R [21]. The pathophysiology of chronic renal injury in these models may involve oxidative stress, alteration in gene expression, loss of peri-tubular capillaries [22], and the haemodynamic and fibrotic effects of angiotensin II [23].
Thus, after AKI, initial chronic damage may be subtle and not be associated with obvious abnormality in serum biochemistry, but still of great prognostic significance. Importantly, management of risk factors may attenuate these pathophysiological processes and slow the progression of CKD.
3.3 Diagnosis of CKD in AKI Survivors
Diagnosis of persistent renal dysfunction as an outcome of AKI can be confounded by many factors hampering our ability to define its nature, extent and significance (Table 3.1). The reciprocal relationship between creatinine and GFR implies that, quite large declines in renal function from a normal baseline can occur with only small rises in steady state creatinine, so that GFR can fall by almost half before creatinine becomes clearly abnormal. This is particularly important when assessing renal outcomes because even if the reduction in total nephron mass is relatively small, this can still trigger slow, progressive decline in renal function. Furthermore, in critical illness, acute falls in creatinine generation rate are observed both in clinical settings [24] and animal models [25]. Such reduced creatinine generation, in turn, decreases both the rate of rise and the absolute creatinine increment after a fall in GFR [26]. Importantly, the largest falls in creatinine generation are associated with greatest illness severity [24]. The decreased ability of serum creatinine to reflect the magnitude of decrease in GFR suggests that some patients may develop ‘sub-clinical’ AKI and then CKD. Neutrophil gelatinase–associated lipocalin (NGAL) a biomarker of renal tubular injury is associated with risk of death and other adverse outcomes even in the absence AKI diagnosis based on serum creatinine [27], suggesting that sub-clinical AKI could be common and clinically relevant. It is uncertain whether sub-clinical AKI is a risk factor for CKD. However, as subtle chronic kidney damage can predispose to progressive renal dysfunction, it is possible that some survivors of major illness could be at increased risk of CKD even in the absence of a formal AKI diagnosis during their illness.
Table 3.1
Impediments to recognition and staging of CKD after AKI
Sub-clinical AKI may go unrecognised during acute critical illness |
Milder interstitial injury after AKI may not be associated with reduction in GFR initially, but still be a risk factor for progressive CKD |
Hyperfiltration and loss of renal reserve may mask reduction in resting GFR, despite being a potential risk factor for CKD progression |
Significant reduction in GFR from a previously normal baseline may not cause creatinine to rise above the normal range, despite underlying nephron loss |
Loss of muscle mass after critical illness and in chronic disease may confound ability of serum creatinine to accurately reflect the severity of reduction in GFR |
Even when the AKI is diagnosed, assessing renal function during recovery can be confounded by pre-existing or acquired alteration in muscle mass, liver function [28] and diet. Steady-state serum creatinine is determined by the equilibrium between creatinine production and creatinine excretion. Many critically ill patients have pre-morbid chronic disease and are likely to have reduced creatinine generation at baseline. The use of creatinine-based eGFR in the general population has recently been the subject of a meta-analysis [29]. The prevalence of CKD rose significantly when cystatin-c, a renal filtration marker less dependent on muscle mass, was used for eGFR estimation, with better prediction of all-cause mortality and cardiovascular death. These results suggest that variations in creatinine generation might confound CKD diagnosis in the general population and that these missed diagnoses are clinically significant. Critical illness is then associated with further profound and progressive loss of skeletal muscle protein [30–32] and muscle thickness [32–34], with a strong inverse correlation between muscle thickness and duration of critical illness [33, 34]. Thus, estimates of renal function after critical illness based on ICU or hospital discharge creatinine can fail to detect significant loss of GFR, and will not be directly comparable to a baseline creatinine.
Even when GFR is accurately assessed, measurements may not represent the extent of chronic kidney damage after AKI. Many patients who have developed chronic renal scarring can have relatively normal GFR for an extended period until overt CKD eventually occurs. It has been speculated that loss of renal functional reserve [35] occurs in early CKD as the kidney lacks capacity to augment GFR in response to demand despite preserved baseline GFR [36]. This may occur when the nephron number is reduced, but total GFR is persevered by glomerular hyperfiltration [37]. Such patients have no capacity to increase GFR as their renal reserve is maximally recruited at baseline. Hyperfiltration may be triggered by neuroendocrine responses to renal damage, including local and systemic generation of angiotensin II, and is associated with glomerulosclerosis and eventual deterioration in renal function. These processes are strongly associated with the development of proteinuria. Irrespective of GFR, microalbuminuria (urinary albumin: creatinine ratio >3.5 mg/mmol) is associated with increased all cause mortality, cardiovascular mortality, progressive CKD, end stage renal disease and risk of new AKI, with increasing risk with more severe levels of proteinuria [38, 39]. Thus, proteinuria is a key prognostic indicator for progression of CKD after AKI at all levels of GFR including apparently normal renal function.
There has been considerable recent research into the development of novel renal biomarkers to accelerate and refine the diagnosis of AKI. However, less is known on how these markers relate to chronic renal outcomes after AKI. Plasma NGAL concentrations at AKI diagnosis do correlate with degree of renal recovery [40] and prediction of renal recovery may be refined by combining markers and clinical risk factors [41]. This suggests that those measures that correlate with renal tubular injury may provide an early prognosis for renal recovery after critical illness. However, existing studies have examined renal recovery as freedom from renal replacement therapy and further research is needed to refine the use novel diagnostics to discriminate severity of CKD in patients who come off, or never need, renal replacement therapy. In addition specific markers of renal recovery or fibrosis may be developed to improve prediction of long-term renal outcomes [41]. Finally, serial measurement of AKI biomarkers may be useful to screen for new AKI during recovery from critical illness allowing measures to minimise subsequent recurrent renal injury, which may have a strong impact on recovery of renal function.
3.4 Primary Prevention of CKD After AKI
In the treatment of AKI, avoidance of recurrent renal injury is crucial to achieving maximal renal recovery, thus the treatment of AKI merges into the prevention of CKD. Use of intermittent haemodialysis for RRT in AKI has been associated with poorer renal recovery than continuous modalities of RRT [42], possibly related to intra-dialytic falls in cardiac output with rapid ultrafiltration, causing renal hypoperfusion and recurrent ischaemic injury [43]. This concern has led to recommendations for the preferred use of CRRT in the treatment of haemodynamically unstable patients with AKI [44]. More controversially, it has been recently suggested that the kidney might be best protected in AKI by pharmacological intervention (ACE-inhibition or Angiotensin II blockade) to reduce glomerular filtration and decreasing renal vascular resistance, thus reducing oxygen demand while increasing renal oxygen delivery, even if this were at the expense of acute need for renal support [45]. While this strategy cannot currently be recommended for clinical use it is a provocative suggestion that warrants experimental investigation.
3.5 Managing CKD After AKI
Given the diagnostic difficulties outlined above, it seems appropriate to consider all survivors of critical illness at risk of CKD. In particular, in patients who had clinically overt AKI, follow-up for development of CKD should be considered, irrespective of apparent degree of renal recovery. CKD in general has well-developed clinical guidelines [7] for monitoring and treatment and in the absence of evidence to the contrary, we should apply these principles to the management of AKI survivors with or at risk of CKD. Follow-up will involve measurement of serum creatinine, blood pressure, urinalysis and cardiovascular risk factors; in some patients formal measurement of GFR may be helpful. AKI survivors should be regarded, as having a long-term risk factor for CKD and continued screening should be considered. Patients with more severe renal dysfunction or other specific risk factors may benefit from specialist follow-up with a nephrologist [46] while others could be followed-up in primary care and be referred back to renal services if required. Treatment of hypertension and modification of cardiovascular risk factors are central to management of patients with or at risk of CKD. In particular, in patients with diabetes or hypertension and proteinuria, ACE inhibitors or A2 receptor blocking agents should be considered, as these may reduce proteinuria and the rate of progression of CKD [47]. Finally, recurrent episodes of AKI are a particular concern resulting in a step-wise loss of renal function, and any follow-up programme should address the prevention and early detection of new episodes of AKI.
3.5.1 Proposed Follow-Up Pathway After AKI
Given the above concerns, we would like to propose a framework for the ideal follow-up of adult patients surviving critical illness complicated by significant AKI (Fig. 3.2). Patients with pre-existing CKD and a new episode of AKI they should at least have CKD criteria re-assessed in 90 days to check for CKD progression, if earlier follow-up is not indicated. As more-severe AKI is more likely to result in earlier progression to CKD, in the absence of other evidence, we propose that patients with AKI-KDIGO stage 2 during critical illness should be considered for a specific follow-up pathway. However, many other survivors of critical illness could be at increased risk of CKD, and specific risk factors for CKD (diabetes, hypertension, cardiovascular disease) may indicate monitoring for new CKD within follow-up of that chronic condition. Thus, all patients with AKI stage 2 or greater should receive follow-up of renal function with early review in those with more severe renal dysfunction at discharge (Fig. 3.2). Other patients should have their CKD criteria re-assessed after 90 days with measurement of serum creatinine, calculation of estimated GFR, measurement of urinary albumin-creatinine ratio, urinalysis for micro-haematuria, blood pressure measurement and renal ultrasound (if not recently performed). At this stage patients may require specialist referral, or be deemed appropriate for primary care follow-up at a frequency commensurate with their level of renal dysfunction [48]. Even in the absence of evidence of CKD at 90 days, survivors of significant AKI should have at least one further check for CKD criteria at 1 year to check for late progression, or indefinite follow-up, depending on the presence of other CKD risk factors.
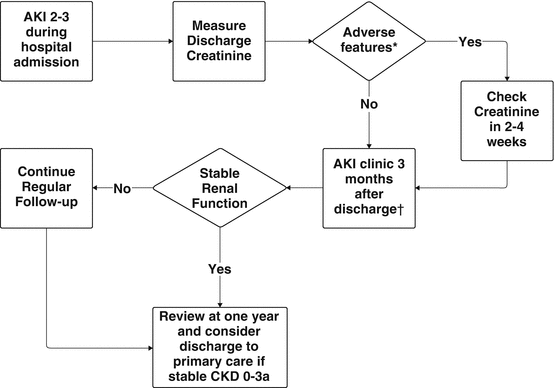

Fig. 3.2
Outline of a pathway for follow-up of patients who survive and episode of AKI 2 or 3 whilst in hospital. * Adverse features suggesting need for early follow-up include a significant increase in serum creatinine from pre-morbid baseline to discharge (new overt CKD or unrecovered AKI) or the presence of significant renal impairment (suggested as a serum creatinine of >175 μmol/L (2 mg/dl) or eGFR <30 ml/min/1.73 m2). Consider formal measurement of GFR or Creatinine Clearance in patients with prolonged critical illness or significant loss of muscle mass. † Patients with specific features including persistent hematuria or proteinuria (Urine Protein: Creatinine Ratio >100), proven or suspected glomerulonephritis, refractory hypertension, familial renal disease, recurrent or extensive nephrolithiasis, or likely progression to ESRD within 1 year should be referred directly to the appropriate specialist nephrology clinic
Systems are required to identify and flag AKI survivors for follow-up. This can be problematic as hospital discharge may be many weeks or months from an episode of AKI and the discharging service may not be the one that actively managed the patient during their critical illness. In the UK, only a fraction of patients who required renal replacement therapy in the ICU and recover renal function receive nephrology follow-up. In addition, AKI is frequently omitted from hospital discharge summaries and clinical coding, as AKI occurs as a complication of major illness rather than as a presenting complaint. Thus, even when seen in other (non-nephrology) specialist clinics, renal function is often not assessed. These experiences are not isolated: in an analysis of 3,929 survivors of AKI with CKD at discharge carried out in the United States, 1 year mortality was 22 %, however, only 8.5 % received nephrology referral before death, need for chronic dialysis, or experiencing an improvement in kidney function [17]. Unless we adequately identify and monitor patients who have had AKI, we will be unable to improve their outcomes. In many cases appropriate long-term follow-up can be accomplished in primary care, but this is only possible if patients are identified and appropriate clinical guidelines are provided by specialists. There is thus a clear impetus for establishing mechanisms to adequately identify and follow-up patients with AKI in particular after critical illness.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





