Fig. 28.1
Colorectal anastomosis with transmesenteric passage of the transverse colon and closure of the peritoneal defect
This procedure was first described in 1961 by André Toupet [17, 18]. At the beginning, the aim of this transmesenteric passage was to perform a tension-free anastomosis between the transverse colon and the sigmoid colon following a left segmental colectomy, with passing the colon on the left of the superior mesenteric artery. The transmesenteric route of the transverse colon was then taken up in 1976, with an opening of the meso created in the right mesocolon, between the right colic artery and the ileocolic artery [19].
In the literature, studies reporting this retro-ileal tunnel are few. In 1978, Turnbull gave the results of 11 patients [20]. There were six patients with complicated, extensive diverticulitis, four patients with colon cancer located to the splenic flexure and one patient with radiation stricture of the descending colon. They all had a resection of the left colon and the distal part of the transverse colon. Postoperative mortality was nil. Early postoperative morbidity was 18 %, with one postoperative ileus managed nonoperatively and one wound infection. Functional outcome was considered good by the authors but not detailed. Hogan and Joyce described a case report of redo surgery using this retro-ileal anastomosis for chronic anastomotic leakage after previous segmental left colectomy [21]. Recently, Sileri and colleagues reported their experience of 10 patients, with two of them operated on by laparoscopy [22]. Indications were the following: two left colon cancers, two left colon cancers with associated diverticular disease, two iterative resections for metachronous left colorectal cancer, two synchronous colon cancers and two patients with extensive diverticular disease. Functional outcome assessment revealed that only two patients routinely used loperamide-based medication. There was no complication related to the surgical procedure, especially the occurrence of small bowel obstruction. Indeed, this point is of importance, as this technique is associated with a theoretical risk of internal hernia through the mesenteric window, and the lowered transverse colon needs to be fixed all around the ileal mesenteric defect with interrupted sutures. Conversely, a too narrow peritoneal opening could lead to colonic obstruction or create an obstacle to venous return.
Procedure 2: The Deloyers Procedure
Another technique is Deloyers procedure. It comprises an isoperistaltic anastomosis between the transverse or right colon and the rectum or anus, after full mobilization and reversal of the residual colon around the axis formed by the ileocolic pedicle (Fig. 28.2). This technique requires also a section of the mesenteric root up to the duodenojejunal flexure. Once the origin of the right colic artery and the middle colic artery are identified, both pedicles are transected and the devascularized colon is resected. The remaining colon (usually including the cecum, the ascending colon up to the hepatic flexure) is then returned in a counterclockwise direction. This craniocaudal trigonometric rotation maintains the cecum in the right iliac fossa, with its anterior surface facing the retroperitoneum, or places it in the right hypochondrium, depending on the length of the remaining colon and the level of the anastomosis.
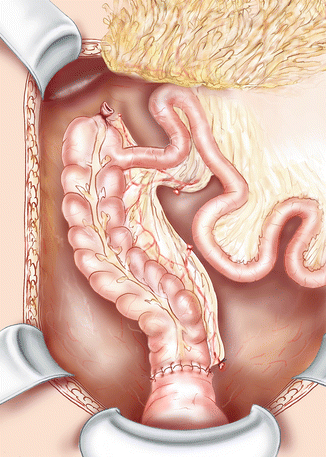

Fig. 28.2
Isoperistaltic colorectal anastomosis after Deloyers procedure with reversal of the colon. The small intestine is repositioned and should not come under the right mesocolon with incarceration
This procedure was first presented by Lucien Deloyers in November 1963 at the meeting of the Surgical Society of Lyon [23]. This Belgian surgeon (1901–1982) detailed this transposition of a colonic segment on a series of 11 patients, aged 17–44 years and operated on between 1956 and 1962. The indications were four ulcerative colitis, three megacolons, three dolichocolons with chronic constipation and one colonic polyposis sparing the right colon. An associated proctectomy was necessary in four of them. There was no postoperative death and according to the author, the postoperative course was uneventful with no need for reintervention. During follow-up, the number of stools per day ranged from one to three.
In the initial description of the technique, the cecum was placed under the liver, in place of the hepatic flexure, with ascending colon occupying the right paracolic gutter and fixed to the parietal peritoneum with interrupted sutures. At control barium enema, the cecum came back most of the time in the right iliac fossa, without this change of position has modified functional outcome.
Since this first publication and during 50 years, only four studies, involving a total of 32 patients, have been published on this procedure [24–27]. All these reports were focused on specific indications such as Hirschsprung’s disease and severe chronic constipation. Prevot reported a series of 7 patients with one postoperative death and good functional outcome in 83 % of cases [24]. The study of Costalat and colleagues included 18 consecutive patients with colonic inertia [25]. In their series of five Duhamel procedures for Hirschsprung’s disease operated on by laparoscopy, Bonnard and colleagues used this surgical maneuver for an infant of 5 months whose disease reached the left half of the transverse colon [26]. Tang and colleagues analyzed the postoperative course of 12 children with a diagnosis of intestinal neuronal dysplasia who underwent extended laparoscopic colectomy with Deloyers procedure and transanal endorectal pull-trough procedure, with two anastomotic leakage (17 %) [27]. Finally, a small retrospective study including three patients gave results of this right colonic transposition technique, with two synchronous colorectal tumors and one unifocal stricturing Crohn’s disease affecting the entire left colon and distal transverse colon [28].
We reported a series of 48 consecutive patients operated on over a period of 12 years [29]. The Deloyers procedure was used as a salvage technique for low CRA or CAA. The main indications were Hartmann reversal, failed previous colorectal anastomosis with anastomotic leakage and chronic pelvic sepsis or chronic stenosis, extensive diverticular disease, left colon cancer, ischemic colitis requiring extended left hemicolectomy and iterative colectomy for colon cancer. In total, 32 patients (66 %) had a previous left colectomy or rectal resection. There was one postoperative death from nonsurgical cause. Thirty-seven patients (77 %) had uneventful postoperative course and no anastomotic leakage occurred. Only one patient required a new diverting stoma because of poor functional results at the end of follow-up. For those whose functional outcome could be evaluated, the median number of bowel movements per 24 h was 3 (range, 1–7) and 82 % had fewer than 4 bowel movements.
All patients were operated on by laparotomy. Although laparoscopic approach is theoretically feasible, it should be noted that this surgery is demanding, as evidence by the median operative time of 415 min in our series. This technique of reconstruction is rarely indicated (3.7 % of all CRA and CAA performed in our institution). However, it always allowed to take down a colonic segment with good blood supply and of sufficient length to perform a tension-free anastomosis in the pelvis or even at the level of the anal margin for 10 patients (21 %). By analogy, in the study of Rombeau and colleagues [20], the transmesenteric lowering of the colon was necessary in 11 of 302 patients operated on for resection of the descending colon at the Cleveland Clinic from 1966 to 1976, which represents 4 % of all CRA in 10 years. Based on these figures, it can be estimated that digestive surgeons need additional tricks to further lengthen the colon in about 5 % of cases.
Before division of the right colic vessels, we recommend to perform a clamping test with a vascular clamp for a few minutes to determine adequacy of blood supply with the ileocolic artery and the marginal artery of Drummond. In our series, an additional colonic resection was necessary for seven patients (15 %) due to the occurrence of ischemia in the terminal part of the remaining colon. Similarly, because appendicectomy would be technically difficult after this procedure, we systematically remove the appendix at the time of surgery.
In 2013, Dumont and colleagues reported a retrospective study of 39 patients operated on for an extended left colectomy with restoration of bowel continuity after either right colonic transposition or complete intestinal derotation with creation of a complete mesenterium [30]. However, the right colonic transposition described in this study was not a typical Deloyers procedure, as the remnant colon was rotated anteriorly at 180° in the sagittal plane, placing the mesocolon of the lowered colon in front of the terminal ileal loop. This could cause small bowel obstruction by compression or, in the event of a postoperative ileus, could place the CRA or CAA under tension [31]. The main surgical indication for an extended left colectomy was intraperitoneal disease (82 % of patients). There was no postoperative death. Postoperative morbidity was 28 %, including three anastomotic leakages requiring reintervention (7.7 %), with no difference between the two groups.
One of the criticisms to this procedure is the risk of vascular kinking of the ileocolic pedicle due to the 180° rotation of the colon. However, we systematically divide the entire mesenteric root, so that the torsion of the pedicle is distributed over a large length. In the study by Dumont and colleagues, this maneuver was not performed and one patient required a total colectomy with an ileorectal anastomosis due to intraoperative ischemia [31].
Procedure 3: Subtotal Colectomy with Cecorectal End-to-End Anastomosis
When the right colon cannot be preserved, the last alternative is subtotal colectomy with antiperistaltic end-to-end cecorectal anastomosis. It allows preservation of the terminal part of the ileum, the ileocecal valve and the cecum, with no visceral rotation or vascular torsion. The entire remaining colon should be completely mobilized. Colonic resection leaves in place only the cecum and the proximal part of the ascending colon vascularized by the terminal branch of the superior mesenteric artery with the ileocolic artery. After systematic appendectomy, the cecum is brought into the pelvis and a cecoproctostomy is then fashioned by anastomosing the base of the cecum to the rectum [32]. In case of mechanical anastomosis, the colonic section line can be used as the entrance for the circular stapler, after introducing the anvil in the rectal stump [33].
Studies that reported results of this technique are few and focused mainly on the surgical treatment of chronic constipation with colonic inertia resistant to medical treatment (after failure of dietary measures and long-term laxative treatment) and confirmed objectively with a colonic transit time study [34–39]. There should not be any sign of terminal constipation on defecography and manometry, and no cause of colorectal obstruction on colonoscopy. Four of these studies were retrospective non-comparative monocentric studies of small effective, with a total of 74 patients [32, 34–36]. The short-term results were judged to be good in all the studies, with no postoperative morbidity or mortality. Three publications came from the same surgical team, with probable duplicate results from the same cohort [32, 34, 35]. A comparative retrospective study of 37 patients evaluated subtotal colectomy with restoration of intestinal continuity using this technique or by ileorectal anastomosis [37]. Overall rate of postoperative morbidity was similar between both procedures (11.8 % after cecorectal anastomosis vs. 10 % after ileorectal anastomosis). However, after a median follow-up of 4 years, functional results were significantly improved for patients with cecorectal anastomosis in terms of mean number of bowel movements per day (2.4 ± 0.9 vs. 3.4 ± 0.8; p = 0.0014), anal incontinence evaluated with the Wexner score (4.3 ± 1.8 vs. 5.8 ± 1.9; p = 0.0223) and quality of life evaluated with the gastrointestinal quality of life index (GIQLI) (119.8 ± 7.5 vs. 111.1 ± 12.0; p = 0.0455). Finally, a retrospective study of 79 patients compared subtotal colectomy with cecorectal anastomosis or ileosigmoid anastomosis [38]. At 12 months postoperatively, the number of bowel movements per week was in favor cecorectal anastomosis (10.2 ± 5.4 vs. 15.5 ± 3.8; p < 0.05). However 26.8 % of patients after cecorectal anastomosis had persistent constipation and routinely used laxatives, versus 6.7 % after ileosigmoid anastomosis (p < 0.05). Similarly, the use of enemas was significantly higher after cecorectal anastomosis (11.8 % vs. 2.2 %; p < 0.05). Overall, the percentage of patient satisfaction was significantly higher after subtotal colectomy with ileosigmoid anastomosis (93.3 % vs. 73.5 %; p < 0.05).
Descent of the Colon Through the Pelvis: The Soave Procedure
Pelvic dissection during salvage surgery for anastomotic complication, and particularly in septic ones, can be of great difficulty and hazardous due to inflammatory phenomenon and fibrous tissues. It can be particularly dangerous for all neighboring structures (pelvic nerves, presacral veins, ureters, vagina). One possible solution to allow the colon to reach the anal canal in a scarred pelvis is Soave procedure. This transrectal coloanal sleeve anastomosis has been originally described by Soave in 1964 for the treatment of Hirschprung’s disease [40]. As reported by Parks, this procedure can be used in case of rectovaginal fistula [41], and also in case of rectourinary fistula [42] or when the pelvis seems completely “frozen” and a perirectal plan of cleavage is not visualized [43].
The pelvic dissection is performed down to the fistula or stenosis located at mid or low rectum. After transection of the rectum and submucosal infiltration with saline-adrenaline solution, the mucosa is excised from the residual rectal stump along the plane of the submucosa and the dissection is continued downwards as far as possible. The patient placed is then placed in the lithotomy and Trendelenburg position to complete the mucosectomy from the dentate line via a perineal approach. After excision of the mucous coat, the colon is then delivered through this rectal muscle tube and a handsewn straight CAA is performed (Fig. 28.3). In case of fistula, the site of the fistula is thus covered by healthy tissues with the colon lowered to the perineum. To facilitate the delivery in case of narrow and fibrotic pelvis, a laparoscopic wound retractor can be placed through the pelvic hole. It allows the colon to slide more easily without excess traction and helps prevent injury of the mesocolon [44]. The residual rectal muscular sleeve must allow the passage of at least three fingers. If this is not the case, the denuded rectum is transected vertically on the posterior wall to increase its diameter.
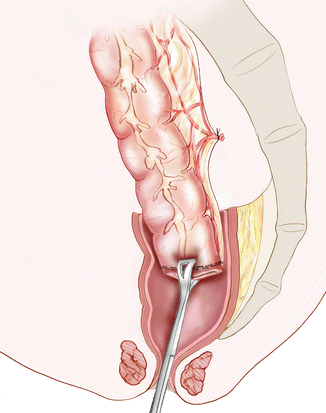

Fig. 28.3
Soave or Parks procedure. A Babcock clamp is introduced through the anal canal to grasp the colon and to gently pull it down in this muscular tunnel. In case of rectovaginal or rectourethral fistula, the lowered colon can be rotated so the mesocolon is placed in front of the defect
Literature Review of Studies Dealing with Redo Surgery for Failed Colorectal or Coloanal Anastomosis
To date, only three surgical teams have reported their experience of redo surgery for failed CRA or CAA, with four retrospective studies and a total of 176 patients (Table 28.1) [3, 45–47]. The first study of Saint-Antoine hospital specially focused on intractable anastomotic stricture [3]. This anastomotic-related complication was also the main indication for redo surgery in the other studies [45, 47].
Table 28.1
Literature review of published series on redo surgery for failed colorectal or coloanal anastomosis
Schlegel and colleagues [3] | Lefevre and colleagues [45] | Pitel and colleagues [46] | Genser and colleagues [47] | |
|---|---|---|---|---|
Study period | 1992–1996 | 1999–2008 | 2000–2010 | 1998–2011 |
Total number of patients | 27 | 33 | 66 | 50 |
Initial disease, n (%) | ||||
Colorectal cancer | 13 (48) | 19 (58) | 52 (79) | 29 (58) |
Diverticular disease | 7 (26) | 11 (33) | 3 (5) | 19 (38) |
Inflammatory bowel disease | 0 | 2 (6) | 0 | 0 |
Others | 7 (26) | 1 (3) | 11 (17) | 2 (4) |
Indications for Redo Surgery, n (%) | ||||
Chronic pelvic sepsis | 0 | 5 (15) | 21 (32) | 14 (28) |
Anastomotic stricture intractable by endoscopy | 27 | 17 (52) | 10 (15) | 20 (40) |
Hartmann’s reversal | 0 | 6 (18) | 13 (20) | 8 (16) |
Rectovaginal fistula | 0 | 0 | 22 (33) | 3 (6) |
Anastomotic cancer recurrence | 0 | 5 (15) | 0 | 5 (10) |
Procedures before Redo Surgery, n (%) | ||||
New anastomosis attempted before RS
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



