Inclusion criteria
Exclusion criteria
Confirmed diagnosis of primary sclerosing cholangitis before liver transplantation
Hepatic artery thrombosis or stenosis
And
Chronic ductopenic rejection
Cholangiographic evidence of intrahepatic and/or extrahepatic biliary stricturing, beading, and irregularities more than 90 days after liver transplantation
Anastomotic strictures
Or
Nonanastomotic strictures less than 90 days after liver transplantation
Histological evidence of fibrous cholangitis and/or fibro-obliterative lesions with or without ductopenia, biliary fibrosis, or biliary cirrhosis
ABO incompatibility between donor and recipient
A diagnosis of recurrent PSC can be made by means of cholangiography revealing nonanastomotic biliary strictures of the intrahepatic and/or extrahepatic biliary tree with beading and irregularity, occurring more than 90 days post-transplantation. However, assessing the bile ducts via the endoscopic route after liver transplantation for PSC is usually not feasible because most recipients have a Roux-en-Y loop rather than a duct-to-duct anastomosis. Given recent and considerable improvement of magnetic resonance imaging technique with its noninvasive nature, magnetic resonance cholangiography (MRC) has become the first choice to evaluate abnormalities of the biliary tract following liver transplantation, instead of percutaneous transhepatic cholangiography. MRC has been validated as an imaging modality to accurately assess the degree of biliary strictures with identification of mural irregularities and diverticulum-like outpouchings specific of PSC [11]. However, in the transplant setting, emphasis must be put on exclusion of other etiologies that can cause similar cholangiographic change. In a published series by Brandsaeter et al., a thorough examination with magnetic resonance angiography revealed a high rate of hepatic artery thrombosis and hepatic artery stenosis that explain some of the biliary stricture after liver transplantation in the non-PSC cohort [12]. Thus, apart from its assistance in diagnosing recurrent PSC, magnetic resonance imaging is of value for identifying a differential diagnosis of vascular complications.
Histopathological findings suggestive of PSC recurrence are identical to those described in the native liver with PSC. The early features in recurrent PSC are characterized by mild nonspecific cholangitis; acute and chronic “pericholangitis” often accompanied by a mild type 1 ductular reaction involving a variable percentage of portal tracts. As the disease progresses, increased ductal proliferation and neutrophilic and eosinophilic inflammation in the portal tract and periportal edema become apparent [13]. Other chronic cholangiopathic features including intralobular foam cell clusters and marked deposits of copper with Mallory’s hyaline in periportal hepatocytes may be visualized [13]. In the late stage, the typical features of fibro-obliterative lesions may be observed with focal loss of medium and small bile ducts. However, similar changes can be seen with other causes of bile duct injuries in the allograft. Such an overlap particularly with chronic rejection questions the validity of liver histopathology as a sole definition of PSC recurrence. Histologically, the diagnostic criteria for chronic rejection are as follows: (1) senescent changes (including cytoplasmic eosinophilia, cell enlargement and multinucleation, uneven nuclear spacing, loss of polarity), affecting a majority of the bile ducts with or without bile duct loss; (2) convincing foam cell obliterative arteriopathy; or (3) bile duct loss affecting greater than 50 % of the portal tracts [14]. In a transplant study by Jeyarajah et al., histopathologic analysis suggests that chronic rejection and recurrent PSC represent a spectrum of indistinguishable disease [15]. However, the distinct difference in clinical outcome, as evidenced by an increased repeat transplantation rate and lower graft and patient survival in PSC recipients with chronic rejection, clearly suggests that they are two distinct entities that require very different treatment strategies [15]. A history of suboptimal immunosuppression and severe or unresolved acute rejection constitute strong arguments in favor of diagnosing chronic rejection. Therefore, a definitive diagnosis of recurrent PSC mandates documentation of the characteristic cholangiographic findings combined with compatible histological features after exclusion of the other possible causes of biliary strictures.
Recurrence of PSC in the hepatic graft was first reported by Lerut et al., in 1988 [16]. Despite controversy that followed shortly after this concept was introduced; the recognition of recurrent PSC is now firmly established in the liver transplant community. The reported cumulative incidence of recurrent PSC has ranged from 10 to 55 % of the transplanted grafts with a median time to recurrence ranging from 8 to 68 months as shown in Table 8.2 [10, 12, 15, 17–25]. The variation is related in part to differences in diagnostic criteria and duration of follow-up. The use and timing of the protocol applied to detect biliary strictures and/or liver histology appears to be the most important factor for the disparity in the reported cumulative incidence of recurrent PSC. Considering that cholangiographic and histologic features of recurrent PSC are not correlated with biochemical indices, protocol liver biopsies, and cholangiography with a magnetic resonance technique may allow systemic and noninvasive evaluation of recipients with possibly full documentation of disease recurrence.
Table 8.2
Cumulative incidence, risk factors, and outcomes of recurrent primary sclerosing cholangitis after liver transplantation
Authors, year | Cohort size | Follow-up period | Cumulative incidence | Risk factors for recurrence | Outcomes of PSC recurrence | ||
|---|---|---|---|---|---|---|---|
5-year patient survival (PSC recurrence vs. non-recurrence) | 5-year graft survival (PSC recurrence vs. non-recurrence) | Re-OLT | |||||
Jeyarajah et al. (1998) [15] | 100 | 21 months (mean) | 18 (18 %) | ACR | 76 % vs. 89 % | 65 % vs. 76 % | 5/18 |
Graziadei et al. (1999) [10] | 120 | 55 months (mean) | 24 (20 %) | NA | Unchanged (86 % vs. 91 %) | Unchanged (79 % vs. 82 %) | 2/24 |
Khettry et al. (2003) [17] | 42 | 24–168 months (range) | 6 (14.3 %) | Recipient-donor mismatch | NA | NA | None |
Kugelmas et al. (2003) [18] | 71 | 14–91 months (range) | 15 (21.1 %) | OKT3 use | Unchanged (92 % vs. 86 %) | NA | NA |
Brandsaeter et al. (2005) [12] | 49 | 77 months (median) | 9 (18 %) | Steroid-resistant ACR | NA | NA | NA |
Campsen et al. (2008) [19] | 130 | 66 months (median) | 22 (16.9 %) | Cholangiocarcinoma before OLT | 45 % without re-OLT | NA | 7/22 |
Cholangitas et al. (2008) [20] | 53 | 11 months (median) | 7 (13.2 %) | Steroid use for ulcerative colitis >3 months post-OLT | Unchanged (85 % vs. 76 %) | NA | 3/7 |
Alexander et al. (2008) [21] | 69 | 50 months (median) | 7 (10 %) | ACR, steroid-resistant ACR, HLA-DRB1*08 | NA | NA | NA |
Alabraba et al. (2009) [22] | 230 | 82.5 months (median) | 54 (23.5 %) | Intact colon at the time of OLT | Decreased in PSC recurrence | NA | 11/54 |
Egawa et al. (2009) [23] | 20 | 63 months (median) | 11 (55 %) | CMV injection within 3 months | NA | NA | 6/20 |
Moncrief et al. (2010) [24] | 59 | 68 months (median) | 15 (25 %) | ACR, CMV mismatch | Unchanged | Unchanged | 4/15 |
Egawa et al. (2011) [25] | 114 | 42 months (median) | 26 (27 %) | High MELD score, first-degree-relative donors, CMV infection, early biliary anastomosis complication | NA | 39 % vs. 74 % | 11/26 |
8.2 Risk Factors Related to Pathogenesis for Recurrent PSC
Reappearance of PSC in the liver allograft suggests that the mechanisms that lead to the initial development of the disease persist after liver transplantation. This would provide a wonderful opportunity to learn about the pathogenesis of the disease. However, factors determining disease development in the post-transplantation situation have been studied only to a limited extent. Several transplant groups have attempted to identify peritransplantation variables that may predict patients who will develop recurrent PSC. The results in general have been heterogeneous. Potential risk factors associated with disease recurrence included recipient age [15], male gender [26], donor-recipient gender mismatch [17], human leukocyte antigen (HLA)-DR1*08 [21], coexistent IBD [20], intact colon before transplantation [22, 26], episodes of acute cellular rejection (ACR) [21, 24], steroid-resistant ACR [12], orthoclone (OKT3) therapy for steroid-resistant ACR [18], maintenance steroid therapy for greater than 3 months post-transplantation [20], the presence of cholangiocarcinoma before transplantation [19], and concurrent cytomegalovirus (CMV) infection in the recipient [24]. The reasons for these discrepant findings among these studies may be due to the small number of patients with recurrent disease as well as the differences in the study design, the diagnostic criteria, and the interesting and confounding variables considered in the regression model.
Although the pathogenesis of PSC remains unclear, epidemiological and laboratory studies consistently indicate that PSC is a complex autoimmune disorder resulting from the interaction between genetic and environmental factors [27] as proposed in Fig. 8.1. In the last decade, there have been major efforts to delineate the genetic architecture of this condition. Recently, genome-wide association studies (GWAS) and immunochip-based studies identified numerous risk loci for PSC that host genes involved in innate or acquired immune responses [28–34], consistent with an autoimmune component to pathogenesis. Also, GWAS have clearly demonstrated that the major component of the genetic architecture of PSC is within the HLA region. To some extent, the genetic findings from non-transplant setting may guide the discovery of interacting and coexisting environmental susceptibility in PSC patients who developed recurrent disease after liver transplantation. The prognostic relevance of the particular HLA genes that confer recurrent PSC after liver transplantation was investigated by many investigators [15, 21]. In a report from the University of Washington transplant group, the overall frequency of the HLA-DRB1*03, DQB1*02 haplotypes among their PSC recipients was higher than that among donor populations, and this confirms that this genotype is more commonly expressed in patients who have PSC [21]. However, there was no difference in the frequency of this HLA haplotype between patients with recurrent PSC and those not having recurrence, and this suggests that the recipient HLA-specific haplotype represents a genetic predisposing factor rather than an antigen for immune recognition in disease development. Interestingly, there was a higher incidence of HLA-DRB1*08, particularly in the absence of HLA-DQB1*04, in their recipients that eventually developed recurrent disease than in those that did not [21]. However, more work is required to confirm candidate genes, to evaluate the functional consequences of risk variants, and to understand how functional changes contribute to disease-specific pathologies. If the association between HLA haplotypes and risk of disease recurrence is validated in further studies, HLA typing would be useful in donor selection as well as to provide valuable prognostic information at the time of transplantation.
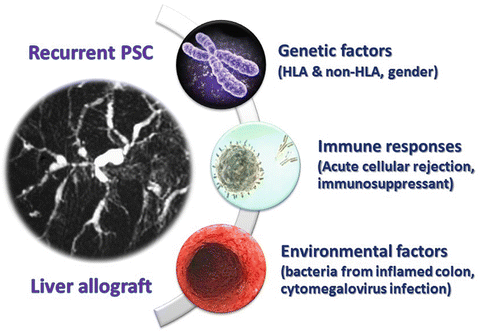

Fig. 8.1
Risk factors related to pathogenesis of recurrent primary sclerosing cholangitis
Of great interest is the reported higher rate of disease recurrence in 114 Japanese recipients of grafts from living-related donors, with recurrent PSC occurring in 32 % at 5 years and 52 % at 10 years after transplantation [25]. A potential explanation may be the first-degree-relatives and sibling have a prevalence of PSC about 100-fold that of nonrelatives [35]. Another possible mechanism contributing to the effect of first-degree-related donors might be linked to the effect of a shared genetic disposition in blood-related recipient and donor pairs including the HLA system. However, the incidence of recurrent PSC in recipients with grafts from related donors other than parents as well as nonrelated donors was similar to those reported for deceased donor liver transplantation [10, 12, 17–22, 26].
The pathogenesis of recurrent PSC could hypothetically be linked to autoimmunity, cross-sensitization between biliary and colonic antigens due to common epithelial epitopes, or leak of bacterial toxins from the inflamed colon with genetic predisposition [36]. The leaky theory is supported by observation of the absence of inflammation in the colon, either due to the absence of concurrent IBD or colectomy before or at the time of liver transplantation has a protective effect against disease recurrence [20, 22, 26]. This was first reported in a study by Vera et al., which demonstrated a dramatic reduction in the risk of PSC recurrence if the colon was removed before or during transplantation [26]. This finding was considerably strengthened in a study by Cholangitas et al., in which no PSC patients without ulcerative colitis or those undergoing pretransplant colectomy developed recurrent PSC [20]. The protective effect of colectomy before or during liver transplantation on the risk of developing recurrent PSC was confirmed in the largest prognostic study of recurrent PSC involved 230 consecutive adult patients who underwent liver transplantation for PSC [22]. Taken together, these findings are consistent with the hypothesis of aberrant homing of mucosal lymphocytes to the liver in the development of PSC [36] that may also be relevant in recurrent PSC. Importantly, these data should not be interpreted as an advocation for pretransplant colectomy but rather as input to understanding the mechanism of developing recurrent PSC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





