5.3 Survival Following Liver Transplantation for PBC
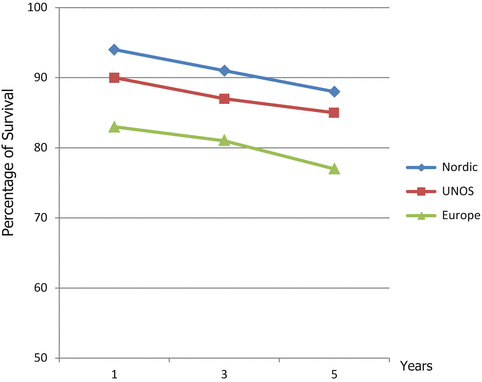
Patient and graft survival for PBC are excellent compared to other indications. At select centers, 1 and 5 years survival rates may exceed 90 % and 80 %, respectively (Fig. 5.2) [8–11]. In addition, PBC patients who are on the liver wait-list have had excellent survival up until recent times. However, it appears that the recent implementation of the Share 35 UNOS Policy may be a contributing factor to the increasing wait-list mortality recently reported in PBC patients [12].
5.4 Recurrent PBC
Recurrent PBC after liver transplant was first reported in 1982 by Neuberger et al. [13]. Despite initial controversy, the recognition of recurrent PBC is now firmly established in the liver transplant community. Unlike PBC in the native liver, clinical and biochemical features cannot be used alone for diagnostic purposes [14–17]. The diagnostic hallmark of recurrent PBC is histologic identification of granulomatous cholangitis or the florid duct lesion [18]. While short- and median-term outcomes remain favorable, long-term follow-up is important to identify potential reduced long-term graft survival in patients.
5.5 Diagnosis of Recurrent PBC
Unlike PBC in the native liver, the phenotypic expression of recurrent PBC is limited. Traditionally related symptoms such as pruritus and jaundice are rarely observed particularly in early recurrent disease. Unlike patients with native PBC, the majority of patients with recurrent PBC have normal or clinically insignificant elevations of serum liver biochemistries at the time of diagnosis. The relationship between change in serum alkaline phosphatase and correlation with histologic disease progression over time remains unknown. Multiple reports have described the persistence of serum antimitochondrial antibody following liver transplantation [19]. A common profile includes immediate loss of detectible serum AMA with subsequent identification in serial investigations. There does not appear to be a correlation between the presence or titre of serum AMA and the development of recurrent PBC.
The diagnosis of recurrent PBC after liver transplantation relies heavily on histologic features. The major diagnostic hallmark of recurrent PBC is granulomatous cholangitis or the florid duct lesions which is present in approximately 40–60 % of initial diagnostic liver biopsies (Fig. 5.3) [18, 20].
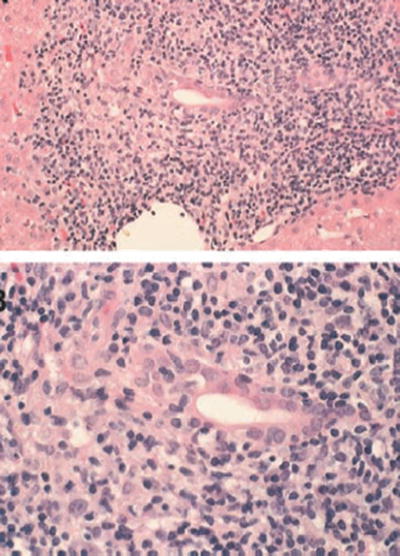

Fig. 5.3
Lymphocytic bile duct destruction (florid duct lesion)
An important relationship between less specific inflammatory features on liver biopsy in patients transplanted for PBC and the influence on eventual disease recurrence has also been identified. In one study, dense lymphoplasmacytic infiltrates occurring before identification of a florid duct lesion was observed in 40 % of patients [21–23]. The diagnostic criteria for recurrent PBC have been outlined by Neuberger et al. (Table 5.1) [21]. In all cases, alternative etiologies for portal tract injury after liver transplant such as acute or chronic allograft rejection, ischemic cholangitis, and drug-induced hepatotoxicity must be excluded.
Table 5.1
Common criteria used for the diagnosis of recurrent PBC include the following
1. OLT performed for PBC |
2. Persistence of AMA or anti-M2 antibody |
3. Characteristics portal triad lesions on a liver biopsy |
(a) Epithelioid granulomas |
(b) Mononuclear inflammatory infiltrate |
(c) Formation of lymphoid aggregates |
(d) Bile duct damage (3/4 definite; 2/4 probable) |
4. Absence of other pathology/disorders, including: |
(a) Acute and chronic rejection |
(b) Graft vs. host disease |
(c) Biliary obstruction |
(d) Vascular abnormalities |
(e) Cholangitis and other infections |
(f) Viral hepatitis |
(g) Drug toxicity |
5.6 Prevalence of Recurrent PBC
Table 5.2 summarizes the prevalence rate for recurrent PBC reported by individual liver transplant programs which range from 9 up to 42 %. There was no obvious relationship between the frequency of recurrence PBC and overall number of patients undergoing liver transplant for PBC. When examined by year or era of liver transplant, the percentage of patients with recurrent PBC is usually increased in more recent times. A number of center-specific issues affect the detection rate of recurrent PBC. The most important factor relates to the use of timing of liver biopsies and follow-up. The performance of liver biopsy for clinical indications alone will underestimate the true prevalence rate as compared to centers that perform protocol liver biopsies. The inherent sampling error of liver biopsies may also contribute to a false negative diagnosis. Finally, the existence of less restrictive histologic criteria used for the diagnosis of recurrent PBC also influences estimates of disease recurrence.
Table 5.2
Recurrence rate of primary biliary cirrhosis reported
Center | Time period | LT for PBC | Recurrent % | Mean time to recurrence |
|---|---|---|---|---|
Pittsburg | 1982–1996 | 421 | 11 | 5.5 |
Birmingham | 1983–2009 | 248 | 42 | 5.1 |
Baylor | 1985–2013 | 250 | 19 | 4.2 |
Mayo Clinic | 1985–2005 | 154 | 34 | 3.5 |
Royal Free | 1988–2008 | 138 | 26 | 3.7 |
Edmonton | 1989–2008 | 108 | 26 | 5.9 |
Berlin | 1989–2003 | 100 | 14 | 5.1 |
Colorado | 1988–2006 | 70 | 26 | 4.8 |
UCSF | 1988–1997 | 69 | 14 | NA |
Washington | 1985–1997 | 56 | 35 | 3.3 |
Kyoto | 1994–2004 | 50 | 18 | 1.6 |
Chicago | 1984–2000 | 46 | 15 | 6.5 |
Lahey Clinic | 1983–2001 | 43 | 19 | 3.5 |
Range | 11–42 | 1.6–6.5 |
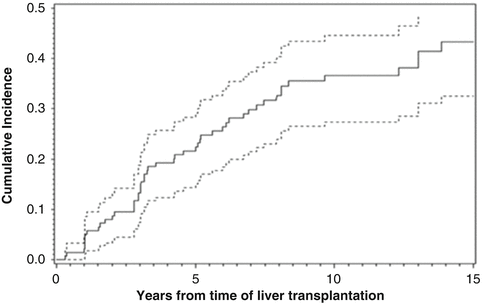
The average time to recurrence varies between reported studies. In general, more cases have been identified when longer duration of follow-up among eligible patients is possible. This is underscored by examples with serial investigations from the same centers report increasing rates of recurrent disease over time. In all, the cumulative incidence rates vary between 21 and 37 % at 10 years, and may be as high as 43 % with 15 years of follow-up. Figure 5.4 shows the cumulative incidence of recurrent PBC over time at the Mayo Clinic.
5.7 Recipient and Donor Risk Factors
Studies attempting to identify risk factors for the development of recurrent primary biliary cirrhosis have yielded conflicting results. Although some studies have identified donor age, recipient age, warm ischemic time, and cold ischemic time as significant risk factors [15, 24–28], others have failed to show significant differences in these factors. Thus, the clinical relevance remains unclear. In addition, other factors such as race, ethnicity, gender mismatch, HLA mismatch, serum bilirubin, INR, and creatinine levels were not significantly different in most studies between patients with recurrent PBC and those without recurrent PBC.
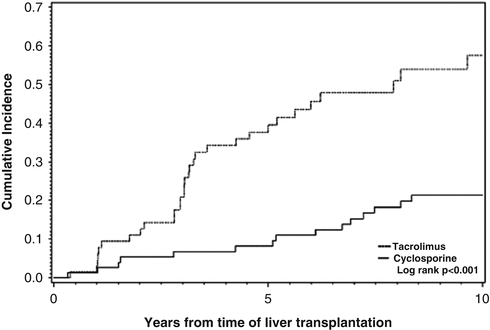
Another area of much deliberation is whether specific immunosuppression regimens used to treat PBC posttransplant can be a risk factor for recurrent PBC. Recurrent PBC was initially reported when cyclosporin was the only calcineurin inhibitor that was being utilized. Subsequently, the use of tacrolimus has been associated with recurrent disease in patients undergoing deceased and living donor liver transplant. Compared to cyclosporin-based regimens, tacrolimus has been associated with an increased incidence and a significant reduction in the time to recurrence of PBC (Fig. 5.5) [29–32]. Tacrolimus immunosuppression was found to be an independent predictor of overall risk of recurrent PBC when addressed in the context of recipient age, number of liver biopsies performed after liver transplant, and duration of follow-up. One recent study has shown a relationship between the type of calcineurin inhibitor utilized and the non-HLA locus (rs62276414), which hosts the IL12A gene [33]. Shown in the Kaplan–Meier plot (Fig. 5.6) is the survival curves for different combinations of calcineurin inhibitors at 1 year and the rs62270414 (IL12A) locus genotype AG and GG. However, it remains unclear how IL2 inhibition when interacted with IL2 and IL12 signaling pathways might influence disease recurrence.