Author/institution
Proposed expanded criteria
Radiology/path
5-year survival
Yao, UCSF [14]
1 nodule ≤6.5 cm, or 2–3 nodules ≤4.5 cm and total tumor diameter ≤8 cm
Pathology
MC—72 %
EC—73 %
Herrerro, Pamplona [22]
1 nodule ≤6 cm, or 2–3 nodules each ≤5 cm
Radiology
Not defined comparison Group—79 %
Roayaie, Mt. Sinai [16]
1 or more nodules 5–7 cm
Radiology
EC—55 %
Yao, UCSF [15]
1 nodule ≤6.5 cm, or 2–3 nodules ≤4.5 cm and total tumor diameter ≤8 cm
Radiology
MC—80 %
EC—82 %
12.2.1 Up-to-Seven Criteria
This system was developed using data from a retrospective analysis involving 36 centers, enrolling 1556 patients with HCC undergoing LT. It includes seven as the sum of the size of the largest tumor (in cm) and the number of tumors, in the absence of microvascular invasion or extrahepatic disease. The 5-year survival rate for HCC within the up-to-seven criteria system was 71.2 % vs. 53.6 % for patients exceeding it. The survival rates were not very different between Milan criteria and up-to-seven criteria. The “metro ticket paradigm ,” was developed as a tool to estimate 5-year overall-survival probability according to size of the largest tumor, number of tumors, and the presence or absence of microvascular invasion. On the basis of metro ticket calculations, as the tumor burden increases from the conventional Milan criteria in a patient, the 5-year overall-survival post-LT decreases. An online calculator was devised to predict the survival post-LT at different intervals on the basis of size of the largest tumor and number of tumors (Fig. 12.1) [17, 18].
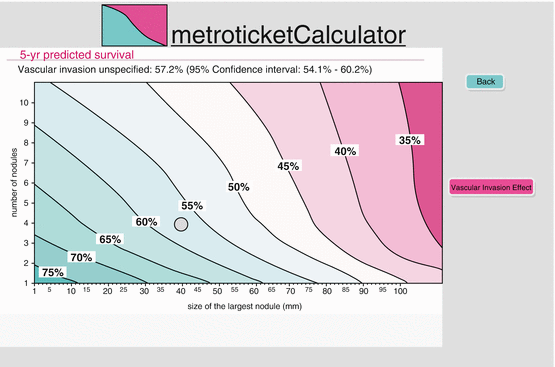

Fig. 12.1
Adapted from metroticket online calculator. Calculated survival is based on size, number of nodules, and the presence of vascular invasion
In light of potential deficiencies of other HCC criteria with regard to difficulties in identifying small lesions accurately in the setting of multifocal HCC, tumor biology not being related to the size and number of the lesions, radiological overstaging, and understaging, the Toronto criteria were designed. In this study, all patients underwent a pre-LT liver biopsy of the dominant lesion. A comparative analysis based on the biopsy results as well as radiologic features of the HCC, and patients were grouped into whether they fell within Milan criteria or within Toronto criteria. Patients with poorly differentiated tumors on biopsy and radiological vascular invasion were excluded and the size and number of tumors did not matter, but AFP more than 400 was considered to be a predictor for recurrence. The 5-year overall-survival rate was 70 % within Toronto criteria vs. 72 % within Milan criteria with similar recurrence-free survival rates. The limitation was sampling error and staging difference at different sites of the tumor [19–21]. Another study performed in a prospective manner utilizing radiological assessment used criteria of 1 tumor nodule ≤6 cm in diameter or 2–3 nodules of up to 5 cm each; patients with vascular invasion and extrahepatic spread were excluded. Patients underwent regular imaging and locoregional treatment as deemed necessary to prevent wait-list dropout. Intention-to-treat survival of the 26 patients who exceeded the Milan criteria when they were included on the waiting list was comparable to survival of those who fulfilled Milan criteria [22].
The increasing wait time on the LT list due to the severe donor organ shortage has led to progressive tumor burden in many patients. The intent-to-treat approach has been addressed, in that patients with HCC waiting for LT for more than 1 year have been found to have reduced overall survival compared to those waiting for less than 1 year. This reduced survival rate has been attributed to an increase in the number of patients whose tumors progressed beyond Milan criteria, causing the LT center to remove these advanced cases from the waiting list. Estimating progression rates is critical for establishing the patient group that should receive the highest priority on the waiting list [23, 24]. Freeman et al. analyzed United Network for Organ Sharing (UNOS)/OPTN data and found that MELD score at listing, maximum tumor size and alpha-fetoprotein level, in addition to age were the only independent factors associated with increased risk for candidates with HCC, dropping off the list [25].
Most of the Asian literature has revolved around LDLT . Many centers in Asia thus follow different criteria, the most studied criteria of which is the “Kyoto criteria ” involving a combination of tumor number ≤10, maximal diameter of each tumor ≤5 cm, and serum des-gamma-carboxy prothrombin (PIVKA II) levels ≤400 mAU/mL. The overall survival rate was 82 %, significantly higher 5-year recurrence rates occur in patients beyond these criteria than within the criteria (9.5 % vs. 7 %). PIVKA II was demonstrated to be more significant than AFP in predicting recurrence [26, 27].
12.3 Patterns and Predictors for Recurrence
A post-LT recurrence may occur when an extrahepatic metastasis has been missed (or was not detectable) during the pre-LT work-up, or it can also be the consequence of circulating HCC cells engrafting and growing in a target organ during the peri-LT period. Given the higher original cancer load of extrahepatic metastasis, such recurrences are expected to appear earlier after transplantation. These two mechanisms may help explain the observed bimodal distribution of recurrences, with most of them appearing during the first 18 months. The time between LT and recurrence is vital in predicting the outcome after recurrence, with worse survival rates occurring when recurrence is diagnosed within 12 months from LT [28–31].
Patients who undergo LT for HCC are always at risk of tumor recurrence. There have been many factors studied that contribute to disease recurrence, and even more so when utilizing the expanded criteria.
Guzman et al. in 2005 utilized immunostaining for p53 and Ki67 in explanted livers and suggested that positive immunostaining of HCC lesions in liver explants was strongly associated with more rapid tumor recurrence after LT. They concluded that patients with staining for both p53 and Ki67 on pre-LT biopsies and who had serum AFP levels >100 ng/mL were particularly at higher risk for early tumor recurrence [32]. Stroescu et al. found that the high labeling index of proliferating cell nuclear antigen (PCNA), p53 nuclear accumulation and high VEGF expression were all associated with a poorer survival in patients with HCC. These data suggest that p53 and VEGF molecular diagnosis along with the expression of proliferating markers (Ki67 and PCNA) may be prognostic markers for recurrence in patients undergoing LT for HCC. It needs to be further studied as to whether these markers can emerge as indicators for vascular invasion and further help in estimation for HCC recurrence [33].
A study from the University of Alberta and University of Geneva used a score for patient selection based on a combination of TTV (Total Tumor Volume ) ≤115 cm3 and AFP ≤400 ng/mL for patients with HCC undergoing LT. It was found that recurrence rates were similar at 10 % vs. 12.8 % for patients within and beyond Milan criteria, respectively, when following these criteria. Their analyses indicated that patients with a TTV greater than 115 cm3 or an AFP greater than 400 ng/mL had significantly worse survival, with a 50 % survival at 3 years and an overall recurrence rate of 12.8 %. TTV was calculated by adding the maximum volume of each HCC, based on a spherical equation formula (4/3πr 3) in which r is the maximum tumor radius of each lesion. In following this method of calculating TTV, it was demonstrated that as tumors increase in size, they frequently deviate from a truly spherical shape, thus more recently an ellipsoid formula has been used that considers diameters in all three dimensions. For example, the volume of a lesion 6.5 × 5 × 4 cm for example would presumably be 144 cm3 calculated using the maximum diameter only with the spherical equation. Using the three diameters and an ellipsoid formula, the actual volume would be 68 cm3, which is a more accurate calculation, and may more precisely predict the recurrence risk.
12.4 Predictors of Recurrence
Smoking per se in the pre- and post-LT setting is an independent risk factor for HCC. Pre-LT and continued post-LT smoking has also been shown to be associated with de novo HCC and recurrence [34–38]. HCC recurrence post-LT has been associated with a poor prognosis and decreased survival. Tumor size and number are crude surrogates for the biologic aggressiveness of HCC. Several centers have evaluated expansion of criteria based on tumor differentiation, genetic profile, and alpha fetoprotein levels . Recurrence is largely attributed to micro- or macro-metastasis from the primary tumor [39, 40].
Biological parameters like histopathologic grading and microvascular invasion are generally not assessed pre-transplant. The size of the tumor per se does not rule out higher tumor grades or microvascular invasion. However, tumor diameter and number of nodules in correlation with the histopathologic grading are predictive of vascular invasion only in HCC larger than 5 cm [41, 42]. Apart from the already-mentioned risk factors for recurrence, Hanouneh et al. emphasized that the rate of tumor growth is another important factor that predicts recurrence. It was observed that the recurrence rate of HCC after LT was higher among patients who exceeded both Milan and UCSF criteria compared with those who fell within them (33 % vs. 5.8 %). However, there was no significant difference in post-LT recurrence between patients outside Milan criteria with tumor growth <1.61 cm3/month from those within it (11 % vs. 5.8 %). Patients beyond Milan criteria with a slow-growing tumor (tumor growth <1.61 cm3/month) experienced less frequent post-LT recurrence than those who exceeded MC with faster-growing tumors (11 % vs. 58 %). Similarly, there was no significant difference in HCC after LT between slow-growing tumors beyond and those within UCSF criteria (17 % vs. 10 %). The rate of recurrence was significantly higher in subjects beyond Milan criteria and UCSF with faster-growing tumors [43].
In the recent past, there has been a lot of work surrounding the molecular profiling of HCC in order to overcome the discrepancy between tumor morphobiology and post-LT recurrence. Studying short regulatory noncoding RNAs, three major micro-RNA (miRNA) clusters have been identified in a training set of 89 patients with HCC- and HCV-related cirrhosis, within a cohort of 165 patients having different underlying liver diseases. Expression levels of three miRNAs representative at each cluster, miR-520g, miR-516-5p and especially, miR-517a, promoted tumorigenesis and metastatic spread in vivo. Moreover, low expression of two other miRNAs (miR-26a, miR-26b) was correlated with decreased post-LT survival [44, 45]. Sato et al. analyzed the miRNA expression profiles in paired, tumor and non-tumor tissue samples from HCC liver resections, and found miRNAs associated with recurrence in both the tumor and corresponding non-tumor tissues. They then classified them into tumor-suppressive miR and oncogenic miRs. While the expression of certain miRNAs was suggestive of tumor-suppressive function (13 types), there were relevant miRNAs in non-tumor tissue samples that were also associated with recurrence (56 types). Another group in a multivariate analysis has shown that high expression of miRNA-203 in tumor tissue was an independent factor for a better prognosis and HCC recurrence-free survival [46, 47].
Long noncoding RNAs have also been studied. These RNAs are involved in diverse cellular processes, including cell-cycle regulation, immune surveillance, and stem cell pluripotency. HOTAIR (long noncoding RNA HOX transcript antisense RNA ) is one such biologically well-studied, long noncoding RNA [48–50]. Yang et al. studied HOTAIR specifically to predict recurrence in HCC post-LT. Upon clinicopathological correlation analysis, segregation of tumor samples of 60 patients who underwent LT into increased HOTAIR expression and decreased HOTAIR expression groups revealed no significant correlation with any single clinico-pathological characteristic, including age, gender, AFP, histopathological grading, tumor number, or tumor size. Furthermore, on univariate and multivariate survival analysis, the 3-year cumulative recurrence-free survival in HCC patients with a high expression level of HOTAIR was significantly lower than those with low HOTAIR expression. Consistently, patients with overexpression of HOTAIR were also prone to earlier recurrence in HCC patients who underwent surgical resection. Lower recurrence-free survival was demonstrated in the patients within Milan Criteria having high expression of HOTAIR , which suggests that size of the tumor alone is not a definitive criterion to exclude patients from LT [51, 52].
A recent retrospective analysis of post-transplant patients from two LT centers in the United States with further validation of the analysis using an HCC database from Indiana University assessed the clinical impact of ischemia–reperfusion injury on post-LT HCC recurrence. Apart from AFP >200 ng/mL and macro-microvascular invasion and tumor characteristics, it was found that cold ischemia time (CIT) >10 h and warm ischemia time (WIT) >50 min were statistically significant independent predictors for early HCC recurrence (<12 months). In addition, an increased number of blood transfusions during the surgery were more predictive for tumor recurrence with this being a surrogate marker of a more prolonged and difficult surgery [53–57]. Samoylova et al. demonstrated that the risk of HCC recurrence was significantly lower for patients with a waiting time >120 days versus patients waiting ≤120 days on the LT list. However, the association between HCC recurrence and the wait time lost its significance over time and was not found to be statistically significant 2 years post-transplant. Among the patients receiving ablative therapy for their HCC, those waiting ≤120 days had a significantly higher risk of recurrence than their longer waiting counterparts in the first year after LT [43, 44]. The importance of wait time and HCC recurrence is an emerging concept in DDLT . This has been the case with LDLT over the years as there practically is no waiting period due to “fast tracking” of living donor cases. In contradistinction, a few recent studies have not shown significant increase in HCC recurrence post-LDLT, so this issue needs further clarification [58–61].
Sharma et al., utilizing a single center retrospective review, demonstrated that the number of HCC lesions and size of the largest lesion were significant predictors for HCC recurrence; they also demonstrated the importance of older donor age in HCC recurrence [62]. Concentrating on the donor factors in tumor recurrence, Parsia et al. reviewed the UNOS database and proposed that along with the tumor factors (microvascular invasion, tumor differentiation), AFP > 500 ng/mL, donor age ≥60 year, and organs of non-local share distribution, when utilized for HCC liver transplant candidates, may convey a higher cumulative incidence of post-transplant HCC recurrence. BMI appears to have no role in disease recurrence [63, 64]. To predict the risk for HCC recurrence after LT, the importance of the vascular invasion in the explant tumor and tumor grade has consistently been demonstrated. Knowing such histological variables is also important in the pre-LT setting [65, 66]. In 2009, Jonas et al., for the first time showed the prognostic significance of DNA index in recurrence of HCC post-LT on pre-LT tissue biopsy. DNA index was determined by Feulgen staining and semi-automatical image analysis of the histograms was obtained from the liver biopsy specimen. Of interest, a group outside Milan criteria (n = 51) with a DNA index ≤1.5 (cut off) had 5- and 10-year overall-survival rates of 72 % and 68 %, respectively. In the multivariate analysis, only DNA index and vascular invasion were identified as prognostic variables for overall survival and recurrence-free survival. In contrast, fulfillment of the Milan criteria and histopathological grading did not reach statistical significance [67].
Immunosuppression is known to represent a significant risk factor for tumor growth [68–70]. Lower recurrence-free survival has been observed in patients who received increased doses of cyclosporine in the first post-transplant year. Cyclosporine dosage given in the first 12 months after LT and pathologic tumor stage were independent prognostic factors in multivariate analysis [71, 72]. One of the reasons for early tumor recurrence may be the result of either previously undiagnosed distant metastasis that had been present before LT, or spillage of cancer cells at the time of surgical manipulation, all being aggravated by the effect of immunosuppression post-LT. Recurrence is more aggressive in the setting of post-LT immunosuppression as compared to patients undergoing resection; it is unlikely for de novo tumors to develop in the liver allograft within a span of 1–2 years. Increased AFP and imaging surveillance post-LT were used as markers to detect recurrence. The presence of microscopic extrahepatic foci of disease in lymph nodes or distant organs at the time of LT, as well as hematogenous or peritoneal tumor dissemination during transplantation, are mechanisms leading to disease recurrence. Bone metastasis typically portend have a very poor survival [73–76].
Roayaie et al. studied 1674 patients who underwent LT over a period of 14 years in order to look at the pattern of HCC recurrence and survival after recurrence. Fifty-seven patients out of 311 (18.6 %) were transplanted for HCC with a wide range in the time from LT to the diagnosis of HCC recurrence, the median being 12.3 months (range 1.5–60.3 months). Median survival from the time of LT was 24.5 months for patients having a recurrence. Survival for patients with recurrence was significantly shorter for patients transplanted with HCC that did not recur. Median survival from the time of recurrence was 8.7 months; it was significantly greater in patients who recurred after the first postoperative year after the LT (22 %) [77]. It was demonstrated that intrahepatic disease was identified in the early recurrence group, whereas more extrahepatic recurrences were diagnosed in the late recurrence group. The most common extrahepatic sites of recurrence included lung, bone, abdominal lymph nodes, adrenal glands, and peritoneum, in decreasing order of frequency [78, 79].
In a clinico-radiological assessment of 150 patients looking for patterns and prognostic factors for recurrence of HCC in a univariate analysis, serum α-fetoprotein level >100 ng/mL, Child Pugh class other than C (patients with more advanced cirrhosis fared better than those with better-compensated disease), the presence of intrahepatic portal venous thrombosis more than three tumors, largest tumor greater than 3 cm in diameter, greater than an 8-cm sum of tumor diameter and viable tumor volume ratio after interventional therapy greater than 10 % of the entire tumor volume were all found to be significant [80]. Multivariate analysis found three factors independently significant for recurrence; the presence of intrahepatic portal venous thrombosis, largest tumor greater than 3-cm diameter, and viable tumor volume ratio after interventional therapy greater than 10 % of the original tumor [81]. No survival difference was noted when patients were followed with pre-treatment diagnostic imaging , either within Milan criteria or within UCSF criteria, suggesting that the accuracy of diagnostic imaging at certain centers was comparable to the review of explant pathology [15, 82, 83]. Analyzing data from 865 transplanted patients over 30 years helped in developing a prognostic model to predict recurrence of HCC post-LT. In addition to the radiological features, there were three significant biochemical markers (alpha-fetoprotein, neutrophil–lymphocyte ratio, and cholesterol levels) [84].
12.5 Down-staging
The term “down-staging” can be defined as the use of any sort of treatment prior to LT in patients who have tumors beyond Milan criteria in order to reduce tumor stage to be eligible for LT . Both resection and locoregional therapy can be used to down-stage and as a bridge to LT. Pre-transplant treatments are also used for patients within T2 criteria in order to prevent progression which could lead to increased wait-list dropout. There is, however, no consensus on whether such down-staging of HCC to within the Milan criteria/T2 followed by LT has a beneficial outcome (Table 12.2). Although several published series have shown good post-LT outcomes in down-staged patients, high AFP levels in these patients are considered to be a predictor for poor outcome and HCC recurrence. Locoregional therapy for the tumor during the waiting period helps in the reduction of AFP levels, halting the tumor progression and possibly leading to an overall better outcome [85–92]. Change in serum AFP levels (defined as a >50 % decrease compared with baseline) after locoregional therapy is useful in assessing tumor response and survival and for assessing lesions that have progressed on imaging studies. LT recipients having HCC with pre-transplant AFP levels >400 ng/mL have a higher tumor recurrence rate [93, 94].
Table 12.2
Selected studies on post-LT outcomes after HCC down-staging pre-LT
Group | Treatment | Patients | Inclusion criteria | Transplanted patients | RFS post-LT | Survival post-LT |
|---|---|---|---|---|---|---|
Chapman et al. [87] | TACE | 76 | Beyond MC | 17 | 50 % at 5 years | 93.8 % at 5 years |
Yao et al. [88] | TACE, RFA, resection | 61 | 1 HCC 5–8 cm | 35 | 92 % at 2 years | 92 % at 2 years |
2–3 HCCs 3–5 cm | ||||||
Total diameter ≤8 cm | ||||||
4–5 HCCs ≤3 cm | ||||||
Total diameter ≤8 cm | ||||||
Otto et al. [98] | TACE | 62 | Beyond MC | 27 | 68 % at 5 years | 73.2 % at 5 years |
Cillo et al. [99] | TACE, RFA, PEI, resection | 40 | Beyond MC | 31 | No recurrence | >90 % at 3 years |
WD or MD HCC | ||||||
Ravaioli et al. [100] | TACE, PEI, RFA resection | 48 | 1 HCC 5–8 cm | 32 | 71 % at 3 years | NA |
2 HCCs 3–5 cm | ||||||
Total diameter ≤8 cm | ||||||
3–5 HCCs ≤4 cm | ||||||
Total diameter ≤12 cm |
It is now well understood that patients who undergo LT with HCC beyond Milan criteria after down-staging do quite well, if time is added as a criterion for LT. This strategy now referred to as the “ablate and wait,” philosophy is used to assess the response of the tumor to locoregional therapy prior to transplantation [95, 96]. As more patients are waiting longer periods of time on the LT list due to the organ donor shortage and more patients are downsized to meet prioritization criteria, they end up undergoing more frequent locoregional therapy . An increased number of embolization procedures may lead to hepatic artery problems ; thus, one should anticipate this post-LT and be keenly aware of the probable complications of ischemic cholangiopathy and hepatic artery aneurysm or stenosis. Patients undergoing therapy with Yttrium [89] radioembolization as well as previous resections for HCC may develop increased adhesions which can lead to a more technically difficult surgery and an increased transfusion requirement. Thus, the clinician should be aware of this especially in patients being downsized [97].
12.6 Management of Recurrence
The incidence of recurrent HCC following LT in different LT centers has ranged from 6 to 56 % [101–104].
Treatment options depend on whether the recurrence of the disease is intrahepatic or extrahepatic . As with HCC of the native liver, the feasibility of surgical resection versus ablation to treat recurrence in the allograft depends on the experience of the team, as well as the size and location of the tumor. While resection may be more applicable to more superficial and larger solitary HCC, ablative techniques may be sufficient and appropriate in the setting of smaller and more deeply situated HCC. While patients who present with disseminated disease are generally not candidates for locoregional therapy, successful surgical salvage has been reported for intrahepatic and/or confined extrahepatic HCC metastasis. Surgical resection has been associated with better survival as compared to nonsurgical approaches with survival of 15.5 vs. 5.5 months, respectively [105]. Chok et al. reported that patients who did not have bone metastasis and who had late HCC recurrence defined as two or more years after LT, had a more favorable 5-year survival with resection of the metastases (71 %) compared with patients who recurred earlier (7 %) [31, 74, 75, 106].
Multi-modality locoregional therapies have been used in the management of HCC recurrence, although the data are lacking on its use in the liver allograft. Ko et al. reported on 28 patients with recurrent HCC who underwent one or more cycles of TACE after LT; the therapy was well tolerated. However, the long-term outcomes were not reported [107, 108]. Systemic therapies in the management of recurrent HCC have had suboptimal results with limited published data. When HCC progression and tumor burden are not amenable to surgical/ablative/locoregional treatments, sorafenib has proven to increase survival; this modest benefit is similar to that described in patients having advanced HCC in the non-LT setting. Recent studies have confirmed sorafenib to be responsible for a benefit in survival with respect to best supportive care in post-LT unresectable HCC recurrences, with an acceptable safety profile and no apparent drug-to-drug interaction with immunosuppressive medications [107–111].
The role of immunosuppression in the recurrence of HCC post-LT has been a topic of discussion, and there is limited consensus on this topic. Retrospective studies have suggested that the risk of HCC recurrence is increased when calcineurin inhibitors (CNIs) such as cyclosporine and tacrolimus are included as part of the post-transplantation immunosuppressive regimen. The exact mechanism for this increased risk is unknown. In contrast, the mTOR pathway is activated in several models of HCC; inhibition of this pathway may reduce cell growth and tumor vascularity. There are data suggesting that immunosuppression regimens that include the m-Tor inhibitor, rapamycin (sirolimus), or its analogs (everolimus) reduce the risk of HCC recurrence as well as the development of de novo malignancies after LT [70, 112–118]. However, recent studies on everolimus (mTORi) show no significant benefit on recurrence of HCC [119]. Sorafenib along with m-TORi was studied as adjuvant therapy for recurrent HCC, but the study reported a few cases of gastrointestinal and cerebrovascular bleeding. Hence, the combination was not advised, whereas sorafenib with other combinations of immunosuppression had no such untoward complication [120–123]. In our experience, the combination of sorafenib with sirolimus has appreciable side effects and is somewhat difficult for post-LT patients to tolerate long term. Although most clinicians try to minimize immunosuppression in the presence of a malignancy, tapering should be judicious and medication levels followed closely, so as to not precipitate acute cellular or chronic ductopenic rejection [124].
Salvage LT is another method of the treatment for post-liver resection recurrence. Limited data suggests the survival rates between the salvage LT and primary LT are similar. In addition, the 1-year, 3-year, and 5-year overall and disease-free survival rates within selected patients were also similar between the LDLT and DDLT in the salvage LT group, which indicates that salvage LDLT is a safe procedure for highly selected patients. HCC recurrence within 8 months after an alpha-fetoprotein level higher than 200 ng/mL and HCC recurrence outside the Milan criteria at salvage LT were independent risk factors for poor recurrence-free survival after salvage LT [125, 126]. Treating the post-LT patient with antivirals for recurrent HBV or HCV may prevent progressive liver disease and the development of cirrhosis. Prevention of the development of allograft cirrhosis will also prevent the development of de novo HCC. Patients with HBV reinfection have been shown to be more likely than patients without HBV to have post-LT HCC recurrence and HCC recurrence itself is a risk factor for HBV recurrence, whereas HBV recurrence is an independent risk factor for recurrence of HCC in the allograft [127, 128].
12.7 The Issue of HCC and LDLT
As the number of patients on waiting lists for LT is increasing, the use of adult-to-adult LDLT may shorten waiting time and possibly decrease wait-list mortality in patients. The offer of living liver donation to adult recipients with HCC has understandably generated controversy with respect to candidate selection, donor risk, and recipient allograft outcomes. In addition, patients with more aggressive tumor biology , who would otherwise drop off the waiting list due to tumor progression, might be “fast-tracked” to transplant without locoregional therapies and this may lead to an increased recurrence rate of HCC post-transplant [129–131]. It has been postulated that the rapidity of liver regeneration can have a stimulatory effect on residual tumor cells , leading to higher HCC recurrence in live donor transplants. A recent animal model supports this hypothesis; this theory of recurrence in LDLT needs more validation however as various transplant groups have noted higher recurrence rates in LDLT as compared to DDLT recipients [132–134].
Bhangui et al. reported no difference in recurrence rates in LDLT vs. DDLT recipients , but did note a trend for poorer outcomes in LDLT recipients whose tumors exceeded Milan criteria. Looking at the survival pattern in LDLT, Vakili et al. reported a significantly improved 5-year survival in LDLT compared to DDLT recipients (81 % vs. 58 %) despite a significantly higher HCC recurrence rate among the LDLT group (29 % vs. 12 %). Improved survival in the LDLT group may be related to the benefits of a superior quality graft due to potentially younger donors and shorter cold ischemia time [135–137]. In a recent report from Korea that studied inflammatory markers in the living donor transplant setting, Neutrophil Lymphocyte ratio (NLR) and CRP (C-reactive protein) were used to assess prognosis post-LT in patients with pre-LT disease exceeding Milan criteria. They developed a scoring system with NLR and CRP, taking into account that inflammation may play a major part in tumorigenesis . In patients exceeding Milan criteria who had an NLR level <6.0 and CRP level <1.0, there was a much higher disease-free survival post-LT [138].
12.8 How HCC in the LT Setting Is Addressed at Our Center
Non-tumor portal venous thrombus is not considered as a contraindication to LT
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





