Fig. 8.1
Two-dimensional treatment dosimetry plan for treatment of a vertebral body metastasis
Higher-level planning includes intensity modulated radiotherapy (IMRT), where the fluence of the photon beam may be manipulated by static or dynamic multi-leaf collimation, to produce a dose distribution more conformal, or shaped, to the tumour position. IMRT is not a process that is applicable to lower doses of RT used for palliative intent.
In order to achieve the planning outcomes on the treatment couch, multiple quality assurance (QA) processes are needed, both at daily and less regular intervals, on the treatment equipment and for the individual patient. The individual patient setup is confirmed, including regular radiographic images. A modern multiple energy linear accelerator is capable of delivering a wide range of photon and electron energies (Fig. 8.2).

Fig. 8.2
An example of a modern linear accelerator (Elekta, Sweden)
8.2.3 General Clinical Considerations
8.2.3.1 Palliative Radiotherapy Versus Curative Radiotherapy
A broad distinction is generally made between curative radiotherapy, where the goal of therapy is eradication of all malignant clonogens to effect cure, and palliative radiotherapy, where the general principle is symptom control, with the lowest effective dose. Symptom control can be achieved at lower radiation doses without the requirement of complete sterilisation of the malignant population. By way of example, pain control of symptomatic bony metastases has been achieved in a high proportion of studies with low doses such as a single dose of 8 Gy, compared to effective curative doses of 60 Gy or more for the same pathological cell population.
Palliative radiotherapy enjoys a number of considerable logistical advantages relative to curative radiotherapy:
Lower total doses with fewer daily treatments per total dose
For example, a typical dose scheduled for brain metastases is 20 Gy in 5 daily fractions at 4 Gy/day delivered over 5 consecutive days
Shorter schedules, leading to easier treatment schedules for less fit patients
Simpler techniques for delivery of radiotherapy allowing a shorter period for treatment planning and thus earlier delivery of treatment
Reduced toxicities (short and long term) due to lower total doses
8.2.3.2 Symptom Management
There are a number of common symptoms due to advanced cancer where palliative radiotherapy has an established role. These symptoms can exist at a variety of sites and be due to either primary or secondary disease. The most commonly encountered symptoms are those of pain, bleeding, obstruction, and compression.
Commonly encountered symptoms where radiotherapy is an effective treatment option include:
Pain from skeletal metastases
Bleeding from a variety of epithelial carcinomas such as within the bronchus, cervix, bladder, or rectum
Obstruction of a lumen such as the bronchus or oesophagus
Compression of normal tissues such as the brain parenchyma, spinal cord, and superior vena cava
8.2.3.3 Primary Versus Secondary Sites
Although the doses of radiotherapy to deliver effective palliation do not differ with respect to the site being either primary or secondary, it is important to consider this aspect when determining the radiation schedule. Patients with incurable but only local disease may benefit from a higher yet palliative dose to deliver more durable symptom control. Generally, the prognosis is better in the absence of significant metastatic burden, and recurrence of symptoms over time is more likely with lower radiation doses. For a terminal patient with symptomatic secondary disease, the lowest effective dose to achieve symptomatic relief is chosen.
8.2.3.4 Pathology Type
Inherent radiosensitivity between different pathological diagnoses influences the radiotherapy dose prescription. Non-Hodgkin’s lymphoma (NHL) and Hodgkin’s disease are particularly radiosensitive malignancies, and very effective palliation can be achieved with low doses. A retrospective study of 54 patients with indolent NHL found a dose of 4 Gy in two doses of 2 Gy each achieved an overall response rate of 81 %, with a 2-year rate for freedom from local progression of 50 %. Complete response rates of 57 and 27 % were achieved in masses <5 cm and >10 cm respectively. Symptom improvement was recorded in 92 % of sites [3]. The significance of such radiosensitivity is achievement of sustained palliation with minimal toxicity impact, such that large abdominal masses, for example, can be managed effectively. The radiobiological basis to explain such radiosensitivity of follicular NHL has been reported as apoptosis of NHL cells and an elicited immune response [4].
Other pathological diagnoses, although without such inherent radiosensitivity, can be effectively palliated with acceptably low doses of radiation, including carcinoma, melanoma, and sarcoma.
Melanoma has historically been viewed as a radioresistant malignancy based in part on early in vivo cell survival data for cultured cells. This resulted in the recommendation of large individual fraction sizes for melanoma, with the clinical basis for this recommendation based on studies with small patient numbers, heterogeneous doses, and tumour sizes and with short follow-up [5]. The impact of large fraction sizes is potentionally greater toxicity to normal tissues. In support of the radioresponsiveness of melanoma to conventional RT, a review of 26 patients with 39 bone metastases found a palliative response rate of 85 %, with fraction sizes of up to 3 Gy effective in 15 of 19 bony lesions [6].
A randomised study of 137 patients [7] comparing a few large doses (32 Gy in 4 fractions of 8 Gy) with a higher number of smaller doses (50 Gy in 20 fractions of 2.5 Gy) demonstrated meaningful responses for melanoma. For a variety of sites, the majority being soft tissue/skin, equivalent response rates of 60 % versus 58 % and equivalent complete response rates of 24 and 23 % were found. Thus, large fraction sizes above 4 Gy are not necessary to achieve palliative end points for melanoma. What is not immediately obvious is that the trial 32 Gy schedule is calculated as a much greater dose to normal tissues (due to the radiobiological impact of large doses), and as such the 50 Gy schedule achieved palliation with significantly less risk to normal tissue. It is also worth noting the response rate that was achieved, which is highly superior to historical systemic cytotoxic therapies.
8.3 Primary Site Palliation
8.3.1 Lung/Respiratory
A systematic review of palliative thoracic RT for lung cancer [8] examined 13 studies with 3,473 patients included. Complete response rates for palliation of haemoptysis ranged from 68 to 73 %, complete response rates for chest pain ranged from 51 to 57 %, and improvement in cough ranged from 48 to 53 %. Total symptom score and survival were both significantly improved in the higher RT dose group. The lack of quality of life assessment in this review is a limitation, but it can be concluded that patients with better performance status have a modest benefit with a longer course of RT [9].
Brachytherapy is an option for palliation of endobronchial tumours where airway obstruction exists. The dosimetric advantage of brachytherapy relates to the avoidance of delivering radiation through normal lung tissue to access the disease site. Thus retreatment of the lung may be better tolerated with reduced lung and adjacent tissue toxicity. A study of 406 patients [10] treated with intraluminal brachytherapy of the bronchus included 324 patients having initial treatment with this approach. Response rates for haemoptysis (82 %) and stridor (92 %) at 6 weeks were maintained at similar levels at 4 months posttreatment. Similarly a smaller study of 76 patients [11] found excellent response rates for intraluminal brachytherapy. Haemoptysis responded in 95 % of cases, with bronchoscopic assessment at 1–3 months finding a total response rate of 87 %. In a series of 175 patients [12] where the vast majority had received previous external RT, 66 % showed symptomatic improvement, with bronchoscopic improvement in obstruction, to at least 50 % of lumen reopened, reported in 78 %. Survival was significantly improved in the symptomatic responders. Brachytherapy is, however, a more resource-intensive treatment than EBRT, requiring bronchoscopic access, and is used less often due to such factors.
Superior vena cava obstruction (SVCO) occurs with a variety of malignancies, but lung cancer is the principal cause. Provided that no airway obstruction/respiratory compromise exists, therapy can be delayed to allow a histological diagnosis to be made in advance of treatment. Effective palliation can be achieved with RT, with a systematic review of lung cancer patients with SVCO [13] finding complete relief of symptoms within 2 weeks in 78 % of small-cell and 63 % of non-small-cell lung cancer. Endovascular stenting was also reviewed, achieving relief of symptoms in 95 % of more than 150 patients stented. Stenting allows rapid relief of symptoms and as such is very useful for severely symptomatic patients, particularly with non-chemotherapy-sensitive pathologies, and relapsed previously treated patients. Chemosensitive malignancies (small-cell lung cancer, NHL, germ cell tumours) are managed systemically, with the expectation of a rapid response.
Reirradiation of locally recurrent lung cancer can achieve effective palliation of symptoms with low rates of high-grade toxicity, based on a review of published studies [14], with a haemoptysis response rate of 83–100 %.
8.3.2 Gastrointestinal
Oesophageal cancer most commonly presents with dysphagia due to partial or complete obstruction. A review of 127 patients [15] treated with RT found an overall improvement in dysphagia in 70 % with maintenance of food passage to death in 54 %. Higher doses of RT achieved much more durable palliation of dysphagia. A randomised comparison of single-dose brachytherapy with metal stent placement included 209 patients with dysphagia of the oesophagus or oesophagogastric junction [16]. Although relief of dysphagia was slower with brachytherapy than stenting, long-term relief of dysphagia and quality of life scoring were better with brachytherapy, with complications more often with stenting. The presence of a malignant fistula with oesophageal cancer is not a contraindication for RT. Successful fistula closure with combined chemoradiotherapy has been recorded in 17 of 24 patients, with 16 returning to oral nutrition [17].
Locally advanced or recurrent colorectal cancer may present with pain, bleeding, and/or obstruction. In a review of 80 patients with a symptomatic pelvic mass due to metastatic colorectal cancer [18], with symptoms of pain (68 cases), bleeding (18 cases), and obstruction (9 cases), RT achieved symptom palliation in 80 % of cases. Median duration of symptom control was 5 months, with significant factors for duration of symptom control being higher RT dose and concurrent chemotherapy. Reirradiation is feasible for rectal cancer. A review of 50 patients reirradiated for rectal cancer found that a dose of 30–39 Gy was well tolerated, with 3-year local control rate of 21 % for patients not receiving further surgery [19].
8.3.3 Genitourinary/Gynaecological
Locally advanced bladder cancer may often present with haematuria. Patients may not be fit for repeated cystoscopic resection, with palliative RT a less invasive option for palliation. In a randomised study of patients unsuitable for radical management of muscle invasive bladder cancer [20], data on 274 patients analysed at 3 months following randomisation to either 35 Gy in 10 daily treatments with 21 Gy in 3 daily doses of 7 Gy found improvement in haematuria at 3 months in 63 % with no difference detected in efficacy or toxicity between the 2 arms.
Cervix cancer may present as locally advanced disease and be unsuitable for curative treatment due to metastatic disease and/or poor performance status. Pelvic symptoms of bleeding or pain are often the presenting symptoms. A systematic review [21] analysed five series using multiple doses of 10 Gy, separated over one or more weeks, for response of symptoms of bleeding and pain, with partial (>50 %) or complete improvement for bleeding of 80–100 % and pain of 50–100 %. Although this review supports repeated large doses for symptom improvement, durations of improvement and toxicities were poorly recorded, and for patients with a better immediate prognosis, a more conventional dose may be preferable.
8.4 Metastatic Site Palliation
8.4.1 Brain Metastases
Brain metastases are a common problem in a variety of malignancies, and more common than primary intracranial tumours, with 25 % of patients with lung cancer developing brain metastases [22]. The central nervous system (CNS) is also a sanctuary site for cytotoxic agents, which is a factor in the development of brain metastases. Commonly patients present with symptoms due to effects of raised intracranial pressure (headache, vomiting) or neurological loss.
Optimal management depends on assessment of a number of factors, including the patient’s performance status, age, and presence or otherwise of extracranial disease. Prognostic classes (classes 1, 2, and 3) have been created based on these factors [23], with validation in a further trial [24].
Neurosurgical resection and stereotactic radiosurgery (SRS) are both options for patients in the better prognostic class 1. Whole brain RT (WBRT) is generally recommended following surgery or SRS. Randomised trials have studied surgery and postoperative WBRT, compared to WBRT alone. Patchell et al. [25] found that surgery significantly improved survival (40 vs. 15 weeks), control of recurrence, and quality of life, with Noordijk et al. [26] finding significantly improved survival (10 months vs. 6 months) also with surgery. Better outcomes occurred with less active extracranial disease. Kocher et al. [27] reported on a randomised study of 359 patients who underwent surgery or SRS for one to three brain metastases and then randomised to WBRT or observation. Adjuvant RT reduced intracranial relapses, but overall survival was similar.
Patients in a poorer prognostic class are not suitable for surgery or SRS and better managed with WBRT or supportive care alone as options. Median survival with supportive care alone is generally 1–2 months, with WBRT increasing median survival to 3–6 months.
Two randomised WBRT studies comparing commonly used schedules [28] assessed neurological symptom relief, finding an overall rate of improvement in neurological function of 47 and 52 % for the two studies. Median duration of improvement for neurological function was 10–12 weeks and overall survival 15 weeks and 18 weeks for the two studies. Partial or complete relief of specific neurological symptoms occurred in 60–90 % of patients. For the different dose schedules studied, none had an advantage with respect to frequency or duration of improvement or survival.
A recent randomised study of higher doses of WBRT in more favourable patients [29] has compared 40 Gy in 20 twice-daily fractions with 20 Gy in 4 daily fractions, with 36 % of patients receiving prior resection of a solitary brain lesion. Late toxicity was uncommon, and no different between the arms. Intracranial progression and salvage surgery or RT were all significantly less frequent in the higher dose arm of the trial, without any survival difference. In conclusion it was commented that for subgroups of better prognosis patients, a higher dose schedule should be considered. A further randomised study of 90 patients [30], with similar treatment arms, also found improved control of CNS progression with a higher dose schedule, but with no survival advantage.
Typically WBRT schedules of 20 Gy in 5 daily doses or 30 Gy in 10 daily doses are prescribed. Treatment is generally well tolerated, with side effects of alopecia and possible fatigue.
Reirradiation may be beneficial for some patients who have a longer progression-free interval after initial WBRT. A review of 86 retreated patients with a median retreatment dose of 20 Gy found a neurological symptom overall response rate of 70 %, with a median survival of 4 months [31]. Less encouraging results were found in a review of 44 reirradiated patients, receiving cumulative WBRT doses of 38–75 Gy, with partial neurological improvement in only 27 %, median survival of 8 weeks, and 3 of 8 brain necropsies demonstrating brain necrosis [32]. Partial brain radiation is better tolerated than WBRT, and the risk of late toxicity is assessed against the risk of neurological loss due to disease progression.
8.4.2 Metastatic Spinal Cord Compression (MSCC)
Malignant spinal cord compression is recognised as an emergency condition due to the irreversible neurological effect of prolonged compression. It is a common complication from a variety of primary cancers, affecting 5–14 % of cancer patients at some stage in the disease course [33], with the clinical outcome dependent on factors of degree, rate and duration of neurological loss, histopathology type, and rapidity of treatment. Patients may present after a period of days or weeks of increasing spinal pain, possibly with a radicular pattern, followed by neurological deficit, featuring as leg weakness and paraesthesia, with urinary retention, or as an acute presentation with leg weakness or paraplegia.
Early diagnosis is important for prognosis [34, 35], with neurological function at the time of diagnosis predicting for the success of treatment for recovery of neurological loss [36]. Magnetic resonance imaging (MRI) is the optimal method of diagnosis (Fig. 8.3), and the possibility of multiple levels of compression requires imaging of the length of the spine. Initial treatment is with glucocorticoids, typically dexamethasone at doses of 16 mg daily, with opiate-based analgesia. A Cochrane meta-analysis of three trials of differing dose levels was unable to determine clinical benefit and optimal dosage [37], with higher doses associated with serious toxicities [38].
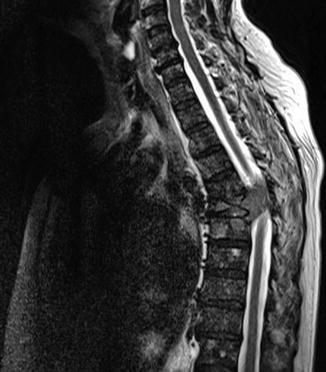

Fig. 8.3
MRI demonstrating extradural cord compression
Treatment options include surgery, radiotherapy, and supportive care alone. Surgery as a treatment option for MSCC using laminectomy with postoperative RT failed to show an advantage compared to RT alone [39]. A randomised study of aggressive surgical debulking with postoperative RT versus RT alone of 30 Gy in 10 fractions [40] found that significantly more surgical patients were able to walk after treatment (84 % vs. 57 %), surgical patients retained the ability to walk for significantly longer (median 122 days vs. 13 days), and significantly more surgical patients regained the ability to walk (62 % vs. 19 %), necessitating early trial closure at interim analysis. In contrast, a matched pair analysis [33] found similar outcomes for improvement in motor function and ambulatory rate post treatment for the surgical and RT alone groups, but with surgical complications an additional factor.
Surgery is a more demanding management approach where patients may already be of poor performance status due to advanced malignancy. The general cancer prognosis and suitability for surgery must be considered alongside other factors of MSCC site, duration of neurological loss, and histopathological type. Radiotherapy is more frequently the sole method of treatment for MSCC. EBRT also produces effective palliation of pain in a significant proportion of patients.
In a randomised trial of two dose schedules with 276 patients with MSCC [41], pain relief (complete or partial) was achieved in 57 %, 90 % of ambulatory patients maintained this level of function, and 35 % of non walking patients regained function. This trial also found a shorter schedule of 2 doses of 8 Gy as effective as an 8 day schedule for response and duration of response. A variety of EBRT dose schedules have been used, and an ongoing randomised trial (SCORAD III) is recruiting patients to either a single-dose schedule or a conventional fractionated schedule.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





