Tools to improve cancer care
Strengths
Weaknesses
Randomised trials
Proof of treatment effects
Selection bias, high costs, time-consuming, confounding factors
Evidence based guidelines/ consensus
Create awareness, focus on key treatments
Rapidly outdated
Meetings and workshops
Create awareness, focus on key treatments
Dependent on speakers/teachers for quality of information transfer
Registry and audit patient characteristics and treatment outcome
Reflect performance and show where improvement can be made
Time-consuming, trouble specifying quality
MDT before and after treatment
Echo of guidelines, reflect on decisions, multidisciplinary communication
Influence of workload/time pressure, dominant persons in a group, team morale
Integrated care pathways
Improve the speed of diagnosis and improve patient communication
Costs to realise the pathway, might not reach individual patient wishes
Patient satisfaction surveys
Reflect on performance
Compliance
Cancer Registries
Since the mid-twentieth century, cancer registries have supplied population-based, comparative survival statistics. The first population-based registry in Denmark that started in 1943 was collected data (1) as a basis for an individual follow-up of patients, (2) for reliable morbidity statistics with a view to an accurate estimate of therapeutic results, and (3) for accurate evaluation of variations in incidence of malignant neoplasms. Other countries followed this initiative by setting up cancer registries.
In 1989, EUROCARE (EUROpean CAncer REgistry-based study on survival and CARE of cancer patients) was funded, based on collaboration between the Istituto Nazionale Tumori (Milan, Italy), the Istituto Superiore di Sanità (Rome, Italy) and population-based cancer registries from 12 European countries, with incidence and survival data available. EUROCARE is a cancer epidemiology research project on survival of European cancer patients [17]. The aims of the EUROCARE project are (1) to describe cancer patient survival in Europe, (2) to disclose whether there are any differences between populations, and if so, how large they are, how they evolve and how reliable the survival estimates [18]. This project was the first to compare cancer survival rates between populations. In the EUROCARE-4 study, colorectal cancer patients diagnosed between 2000 and 2002 demonstrated a mean 5-year relative survival of 56.2 %. However, there was large variation in survival among European countries. Especially North and Central Europe showed best survival rates, while survival rates in the Czech Republic and Poland were substantial lower (45.2 % and 46.0 % respectively) than average [19]. In the most recent EUROCARE-5 analysis, 5-year relative survival increased for rectal cancer with a similar variation between European regions [20].
Comparing survival of patients diagnosed with cancer between different populations is difficult to interpret, as more prolonged survival may depend on later death or earlier diagnosis (adding “lead time”). In order to be able to compare survival rates between different registries, standardised information on disease stage at diagnosis, on diagnostic procedures used for staging, and on treatment decisions are necessary. These items are usually not available from population-based cancer registries.
European Audits
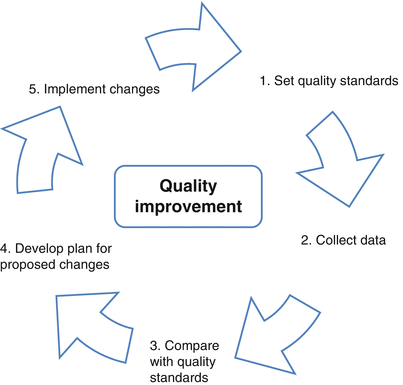
Auditing is an effective instrument to monitor the quality of care and to improve outcome, and can be defined as systematic and independent check-up of outcome data of patients undergoing certain procedures followed by feedback on performance (Fig. 27.1). This way, health care professionals get continuous feedback, own practices will be compared with selected quality standards. The identified gaps provide opportunity for continuous quality improvement. To achieve this, health care providers need to specify clinical endpoints that define high quality care most appropriate. Measuring these endpoints can be challenging in practice. For example, we do know that patients with less than ten nodes found have worse outcomes in general, and we know that more lymph nodes are found if more are requested by (inter-)national recommendations [21].
Since 1993, several European countries initiated a surgical audit. Most audits were initiated for rectal cancer, because of poor outcomes and variation in outcomes between hospitals and individual surgeons. Later, most rectal cancer audits expanded with registry of colon cancer outcomes. Currently, there are eight surgical (colo)rectal audits in Europe (Table 27.2). The Norwegian Rectal Cancer Project started in 1993, and is the first initiated audit. Outstanding results were achieved after 4 years: the share of applied TME surgery increased from 78 to 92 % and the local recurrence rate dropped from 28 to 7 % [13]. Moreover, auditing appeared to be cost effective. Currently, the audit is called the Norwegian Colorectal Cancer Project. The Swedish Rectal Cancer Registry (now: the Swedish Colorectal Cancer Registry), an audit for rectal cancer patients, also demonstrated remarkable results [22]. More than 97 % of the patients with invasive rectal cancer were recorded. According to the Swedish healthcare system, pathologists and surgeons are obliged to report cancer diagnoses to the Swedish Cancer Registry. Between January 1995 and December 2003, 13,343 patients treated for rectal cancer were registered. Postoperative mortality declined under 2.5 % and the 5-year local recurrence rate was 9.5 % [22].
Table 27.2
National audits
Country | National/regional date of launch | Name | Website |
|---|---|---|---|
Norway | National 1993 | Norwegian Rectal Cancer Project | |
Denmark | National 1994 | Danish Colorectal Cancer Group (DCCG) database | |
Sweden | National 1995 | The Swedish Colorectal Cancer Registry | – |
Italy | 1999 | STORM | |
Germany, Poland, Lithuania, Naples | 2000 | International quality assurance in colorectal carcinoma | – |
UK | 2001 | National Bowel Cancer Audit Programme | |
Spain | 2006 | The Spanish TME project | – |
Netherlands | 2009 | Dutch Surgical Colorectal Audit |
Furthermore, rectal cancer survival improved from 36.1 % in the period 1960–1964 to 57.6 % in the period 1995–1999 in Sweden [23]. It is noteworthy that survival of rectal cancer patients even exceeded colon cancer survival, whereas considerable improvements were made by implementation of adjuvant therapy for colon cancer patients during that period, while this did not have an effect on rectal cancer patients. This demonstrates the benefits of structural surgical training and feedback. After the excellent results of the Norwegian Rectal Cancer Project, several other countries have initiated audits on (colo)rectal cancer (Table 27.2).
Although all national audits showed remarkable results, variation in outcomes between and within European countries still exists. To reduce variation within Europe, the EURECCA project has been initiated. EURECCA is the acronym of European Registry of Cancer Care (or in short European Cancer Audit) and is initiated to form a European platform for sharing data of registries and audits to learn from each other, as well as to form a core dataset in Europe. EURECCA Colorectal aims to improve colorectal cancer care in Europe by harmonising and standardising cancer management. Furthermore, subgroups of patients, such as older patients are often excluded from trials. With large population-based studies, evidence can also be obtained for these subgroups. Moreover, for certain topics, as for example omission of surgery in rectal cancer patients with a complete remission after neoadjuvant chemoradiation, it is not possible to set up a trial. By developing a uniform worldwide database of all these patients, we will get insight in the value of the ‘watch and wait’ approach after a complete remission.
Quality Indicators
Safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity, are the six quality concepts that are nowadays implemented in health care organisations. To improve the quality of care, measurement plays an important role and can be done by using quality indicators. This way, potential problems that might need attention can be identified. Quality indicators are defined as ‘measurable characteristics of care that reflect the quality of care’ and serve as standards by which structures, processes, or outcomes of care can be measured. Structural indicators include information on the hospital organisation, while process indicators describe medical care, and outcome indicators focus on outcomes as for example mortality and morbidity. Process indicators have the advantage that data are mostly present in existing databases and that the influence of case-mix is limited. However, outcome indicators are clearer in their relation with quality of care, but are affected by case-mix factors (for example gender, age, ASA classification, Charlson comorbidity index, and previous operations) for which one needs to adjust. Without adjusting for case-mix, hospitals with the sickest patients can incorrectly be stated as the worst hospitals.
Quality indicators are preferably based on scientific evidence, but when limited or weak evidence exists, they need to be developed based on consensus within expert panels (for example by using the ‘Delphi’ method). Quality indicators that are based on scientific evidence have content validity and indicators that are based on consensus have face validity. Defining measurable characteristics can be a challenge, just as developing a ‘perfect’ quality indicator. However, a good quality indicator must at least have been tested for feasibility, reliability, acceptability, validity, and sensitivity to detect changes.
More specific for rectal cancer, examples for process indicators could be:
The amount of patients discussed within multidisciplinary team meetings
The amount of patients with adequate tumour staging by MRI of the pelvis before resection
The amount of patients of which the circumferential resection margin (CRM) of the resection specimen was described in the pathology report [24].
Traditional outcome quality indicators for rectal cancer care were for example survival and recurrences. Nowadays, endpoints as quality of life or functional outcome might be more important for patients than surviving rectal cancer with many invalidating complaints, such as diarrhoea, urinary incontinence, and sexual dysfunction. This shows the importance of assessing and reassessing every quality indicator at a regular basis to evaluate if it still is the most appropriate indicator to represent quality.
Guideline Formation
Another essential component in order to improve the quality of cancer care is guideline formation. Guidelines are needed as a basis for health-care professionals in treating rectal cancer patients. However, since science rapidly evolves, it must be taken into account that guidelines are not always completely up-to-date.
Recommendations in guidelines must be based on highest available evidence. Randomised trials are needed to test hypotheses under experimental conditions, while large cohort studies are necessary to translate the use of a certain treatment on population level. If no evidence is available, agreement on recommendations can be based on expert opinion. An expert panel needs to consist of representatives of all disciplines involved in rectal cancer care. Moreover, especially in international guidelines, the representatives within the expert panel must be equally distributed between the different countries.
To avoid potential bias, the methodology to achieve consensus by voting on statements is of utmost importance. One of the methods that can be used is the ‘Delphi’ method, followed by discussion within the consensus group and further voting rounds. After a consensus meeting, the level of evidence (I-IV) on which the final statement is based must be provided, as well as the level of recommendation (A-D), the level of agreement, and the percentage of disagreement.
In December 2012, the latest multidisciplinary consensus meeting for colorectal cancer, using the ‘Delphi’ method, was held [25]. Besides the actual listing of topics and their rating, experts who attended the consensus meeting wrote reviews to discuss the main points of recommendations [21, 25–28].
Multidisciplinary Team
Multidisciplinary team management will enhance cancer care in many ways. It is the reflection of adherence to guidelines and the rebuttal of non-adherence to guidelines. Moreover, it gives interaction between the disciplines with field specific expertise. The increasing complexity of cancer management and the many specialties involved in the treatment encompass the danger that potentially sub-optimal care could be given. In an ideal situation all patients would receive optimal treatment from an expert specialist team, coordinated by one case officer.
Multidisciplinary Team
Radiation oncologist
Medical oncologist
Gastroenterologist
Surgeon
Pathologist
Radiologist
Nurse specialist
Multidisciplinary care has become an integral part of cancer care in many western countries. Multidisciplinary team (MDT) meetings were organised to warrant that care delivery is consistent with the best available evidence from all different disciplines. The presence of different specialists means a consideration of the full range of therapeutic modalities available for each patient. All new patients with cancer should be presented in a MDT meeting. Patient characteristics, staging and proposed treatment need to be discussed. All restaged patients should be discussed again, as well as all operated patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree