Type I
Macroporous prosthesis pores >75 μ
Monofilament polypropylene mesh
Type II
Macroporous prosthesis (at least one of three directions)
Pores <10 μ
ePTFE mesh
Type III
Macroporous prosthesis with multifilament component or microporous
Polyester mesh
ePTFE perforated mesh
Monofilament polypropylene mesh
Type IV
Prosthesis with submicron pores
A new classification of the meshes for hernia surgery takes into account integration and peritoneum formation (Table 10.2) [6]:
Table 10.2
Classification of prostheses used in the repair of abdominal wall hernial defects
Reticular prosthesis | |
Nonabsorbables | Polypropylene (high or low density), polyester |
Partially absorbables | Polypropylene/polyglactin 910, polypropylene/poliglecaprone |
Absorbables | Polylactic, polyglactin 910 |
Laminar prosthesis | |
Nonabsorbables | ePTFE, silicone, polyurethane |
Absorbables | Porcine intestinal submucosa, bovine pericardium, human acellular dermal matrix, other biological meshes |
Composite prosthesis | |
Nonabsorbable components | Polypropylene/ePTFE, polypropylene/polyurethane |
Absorbable components | Polypropylene/polyethylene glycol, polyester/polyethylene glycol, polypropylene/hyaluronic acid, polypropylene/polydioxanone/cellulose |
The reticular prostheses are useful for placement in a tissue interface.
The laminates are optimal prostheses for placement in direct contact with the visceral peritoneum.
The composite prostheses can be placed on all interfaces, but their design is devised in order to be placed in a tissue and a visceral peritoneum interface.
Laminar Prostheses (Fig. 10.1a–c)

Fig. 10.1
(a–c) Laminar meshes. (a) Dual Mesh™ (L. Gore & Associates, Newark, DE, USA). (b) Omyra™ Mesh (Braun, Tuttlingen, Germany). (c) Mycromesh™ (L. Gore & Associates, Newark, DE, USA)
These meshes provoke low formation of new peritoneum but high integration with the receptor tissues:
Nonabsorbable: PTFE, silicone, polyurethane
Absorbable: biological material (dermis or submucosa of swine, bovine, or human tissue)
A new version of the last Categorization of Coda is used today, its author is Klinge. This classification indicates that the most important is the pore meshes [7].
Coda’s classification |
1. Ultralight ≤35 g/m2 |
2. Light C 35–70 g/m2 |
3. Standard C 70–140 g/m2 |
4. Heavy C ≥140 g/m2 |
Klinge’s classification |
Class I: Large pore meshes (characterised by a textile porosity of >60 % or an effective porosity of >0 %) |
Though the relevance was not clear yet, we further subgrouped for |
(a) Monofilament |
(b) Multifilament |
(c) Mixed structure or polymer (e.g. absorbable + non-absorbable, or different non-absorbable) |
Class II: Small pore meshes (characterised by a textile porosity of <60 % and without any effective porosity) |
(a) Monofilament |
(b) Multifilament |
(c) Mixed structure or polymer |
Class III: Meshes with special features |
Class IV: Meshes with films |
Class V: 3D meshes |
Class VI: Biologicals |
(a) Non-cross-linked |
(b) Cross-linked |
(c) Special features |
Reticular Prostheses (Fig. 10.2a, b)
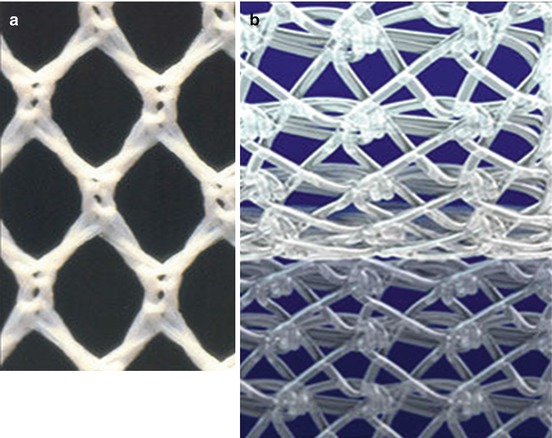
Fig. 10.2
(a, b) Reticular meshes. (a) Polyester. (b) Polypropylene
These meshes provoke high formation of new peritoneum and normal integration:
Nonabsorbable: polyester, polypropylene.
Absorbable: polyglactin, polyglycolic acid.
Lightweight prostheses: these meshes have big pores and low density; can be one single material or mixed materials such as nonabsorbable and absorbable material together.
Composite Prostheses (Fig. 10.3a–c)
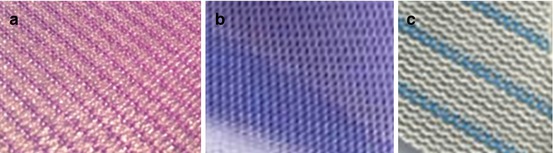
Fig. 10.3
(a–c) Composite meshes. (a) SEPRAMESH™ (Davol, Warwick, RI, USA). (b) Parietex™ mesh (Covidien, Dublin, Ireland). (c) Proceed™ (Ethicon Endo-Surgery, Blue Ash, OH, USA)
These prostheses provoke high formation of peritoneum and high integration. They consist of two different material layers: the first (superior layer) prosthesis is a reticular type, designed to increase the fibroblastic reaction (usually polypropylene or polyester). The second component (inferior layer) is a laminar type, and it can be of absorbable or scarcely reactive material such as the PTFE.
Laminar and composite prostheses are the main meshes used in laparoscopic ventral hernia repair, where the biomaterial is in contact with the visceral peritoneum and the mesh is in contact with the intra-abdominal organs (small bowel, omentum, etc.).
Nonabsorbable Prosthesis [1–6, 8, 9]
Polyester (Dacron)
This is derived from terephthalic acid and ethylene glicol. It was first used in the USA in 1954 as a prosthesis in vascular surgery and subsequently introduced in 1967 in Europe (in France by Rives) for surgery to repair abdominal wall defects. Dacron prostheses (Type III, according to Amid) used for hernia surgery consist of very fine braided polyester filaments. These filaments are light, soft, flexible, slightly elastic, and endowed with high tensile strength.
Their plasticity makes them adaptable to different anatomical situations and suitable for retromuscular or preperitoneal placement during hernioplasty (Rives-Stoppa-Wantz method). Macroporous structure stimulates fibroblast reaction and vivid and rapid formation of a periprosthetic capsule and offers excellent biological tolerance; it does not allow contact with viscera, which would result in high risk of erosions, fistulae, and adhesions.
Dacron prostheses have a lower resistance to infection due to their status as multifilaments, but the fact is that, in case of infection, it is usually necessary to consider timely appropriate antibiotic treatment if a decision is made to remove these types of meshes.
Polypropylene
This is a synthetic polymer derived from polyethylene. It presents certain advantages such as high tensile strength, the ability to sterilize, tolerance to infections and many chemicals, and ease of use. This versatility allows it to adapt to different situations for a tension-free hernioplasty. This biomaterial is hypoallergenic, hardly induces tissue reaction, and generally is well tolerated by patients. This is a Type II and a Type III prosthesis according to Amid.
Since the start of its use in 1958 (by Usher), polypropylene has become the material used in the repair of abdominal wall defects, especially for the treatment of herniated inguinal.
In ventral hernia repair, it is used only in transabdominal preperitoneal hernioplasty.
All polypropylene meshes (Type I according to Amid) have in common a high tensile strength, encouraging rapid intra- and periprosthetic fibroblastic reaction and resistance to infections. Some examples are meshes (PHS®, PAD®, Ethicon Endo-Surgery, Blue Ash, OH, USA) and prefabricated blocks (plugs) (PERFIX®, Davol, Warwick, RI, USA; Hernia Plug Mate System, USSC; Premilene Mesh Plug ®, Braun-Dexon, Tuttlingen, Germany; Self-Forming Plug, Atrium Med Corp, Hudson, NH, USA). Due to the polypropylene forming dense scar tissue and inducing a fibroblastic reaction, it is recommended that these meshes do not have contact with intraperitoneal structures; there is high risk of adhesion formation with possible evolution to erosion and intestinal fistulae.
Polytetrafluoroethylene (PTFE) and Compressed or Expanded PTFE (ePTFE, cPTFE) (Fig. 10.4)
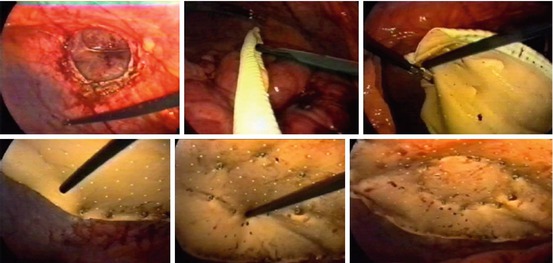
Fig. 10.4
Double-crown technique with Dual Mesh™ (L. Gore & Associates, Newark, DE, USA)
Expanded polytetrafluoroethylene (ePTFE) is a synthetic polymer derived in 1963 in Japan from Teflon and subsequently redefined by Gore in 1970 in the USA. PTFE-e was initially used in surgical prostheses for vascular surgery. In 1983, it began to be used to repair abdominal wall defects as Gore-Tex® Soft Tissue Patch (STP) (L. Gore & Associates, Newark, DE, USA).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








