This article provides an overview of imaging modalities that aid in diagnosing, staging, and assessing therapeutic response in prostate cancer. Prostate cancer is the second most common type of cancer in American men and the second leading cause of cancer death among men. Prostate cancer is difficult to diagnose in early stages, and advanced disease often recurs after treatment. To localize sites of recurrence many imaging modalities have been used with varying success. This article presents case studies of PET scanning using carbon 11 acetate and discusses intravenously infused ascorbate, a complementary and alternative medicine therapy for prostate cancer.
The problem
Prostate cancer is the most common type of cancer found in American men other than skin cancer and is the second leading cause of cancer death among men—second only to lung cancer. For 2010, the American Cancer Society estimated that there were approximately 220,000 new cases of prostate cancer diagnosed in the United States and approximately 32,000 men died of the disease. These statistics suggest that 1 in 6 men will be diagnosed with prostate cancer during their lifetime (1 in 5 for African American men) and 1 in 36 will die of this disease—statistics that are strikingly similar to breast cancer statistics for women. Alarmingly, the general population is unaware of these risks because men are reluctant to talk about their disease or go for annual examinations. In many ways, men still have a denial mindset similar to the mindset women had toward breast cancer before former First Lady Betty Ford’s efforts to destigmatize breast cancer. In 1978, Mrs. Ford publicly acknowledged her breast cancer diagnosis and subsequent mastectomy. Her declaration of her diagnosis and ensuing determination to publicly fight breast cancer encouraged early detection and inspired thousands of American women who were also coping with the disease. American men have not had a similar advocate for prostate cancer step forward and raise public awareness to this same level. This unfortunate disparity in awareness is reinforced by disparate levels of funding for breast cancer and prostate cancer at the National Institutes of Health. In 2010, $824 million was dedicated to breast cancer research whereas less than half that amount—$362 million—was dedicated to prostate cancer research.
Aside from these public health concerns, the problem is compounded by the elusiveness of diagnosis and localization. Early prostate cancer usually has no symptoms. Prostate-specific antigen (PSA) screening can usually detect prostate cancer years earlier than it would be detected by a digital rectal examination or the development of symptoms. Although there is no absolute cutoff between a normal and an abnormal PSA level, screening programs in the United States have commonly used greater than 4 ng/mL to define a positive test. PSA screening, however, has several limitations. Many men who do not have prostate cancer screen PSA positive, whereas some men with biopsy-proved prostate cancer do not have elevated PSA levels. To evaluate the efficacy of screening the at-risk male population, 2 large randomized trials of prostate cancer screening with PSA testing have been completed. The results are conflicting. The United States–based Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial did not observe a correlation between screening and decreased prostate cancer mortality, whereas the European Randomized Study of Screening for Prostate Cancer demonstrated a 20% reduction in prostate cancer mortality among men who were screened for PSA compared with those who were not screened for PSA.
Whether the diagnosis of prostate cancer is suggested by elevation of PSA or by physical findings on digital rectal examination, the initial diagnosis is confirmed by biopsy, often guided by transrectal ultrasound (TRUS). Of the 220,000 new cases diagnosed in 2010, approximately 75%, or 165,000 patients, will choose to have potentially curative therapy—radical prostatectomy or radiation therapy. Unfortunately, these therapies will fail in approximately 40% of these 165,000 patients, or approximately 66,000 men, in whom PSA levels will rise in the following years, indicating recurrence of tumor. The challenge then is to localize the recurrence at an early stage to try to arrest the disease with radiation therapy or surgery (salvage therapy) to possibly effect a cure rather than simply relegate patients to palliative hormonal therapy, which is expensive, carries significant side effects, and ultimately fails in almost all cases.
Many different imaging modalities have been used with varying success to try to localize sites of recurrence ( Fig. 1 ). Most commonly, patients with a rising PSA undergo CT imaging of the abdomen and pelvis along with a radionuclide bone scan. Ultrasound, MRI, indium 111 ( 111 In) capromab pendetide (ProstaScint) single-photon emission computed tomography (SPECT), fluorodeoxyglucose F 18 positron emission tomography ([ 18 F]FDG-PET or FDG-PET), and carbon 11 acetate PET ([ 11 C]acetate-PET) or [ 18 F]choline-PET, however, can be used effectively to help localize recurrence in various clinical presentations. This article provides a brief overview of each imaging modality and its potential applications and limitations.
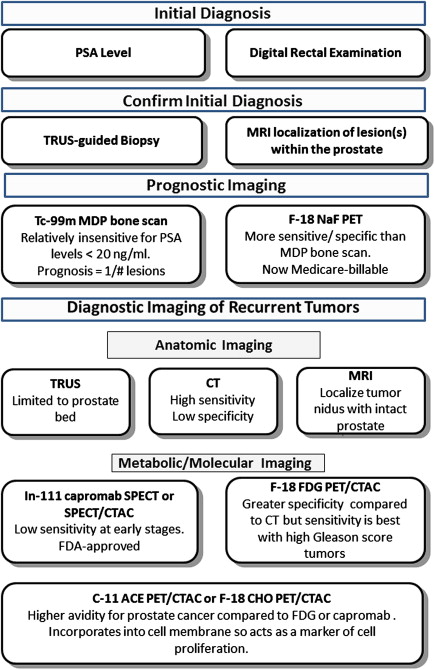
Bone scan
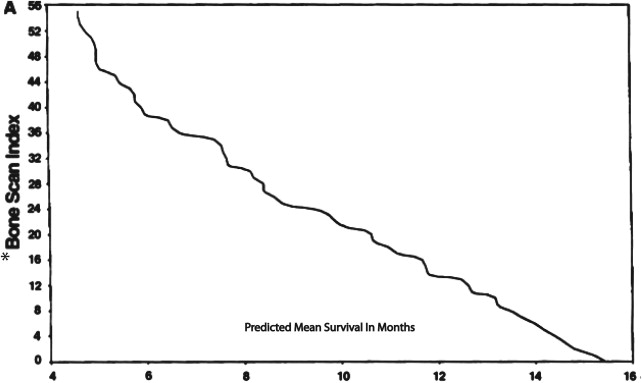
Bone scanning has been used to look for osseous metastases in prostate cancer since the introduction of the modern Anger gamma camera to nuclear medicine in the 1960s. Bone scans are more sensitive for detecting osteoblastic metastasis than are planar radiographs because 30% of the bone matrix must be affected for metastases to be detectable by conventional radiography. Studies dating back to 1976 have shown that at least 62% of patients with a positive bone scan have normal planar radiographs. Bisphosphonates are labeled with radioactive technetium Tc 99m ( 99m Tc) to make a radiopharmaceutical ( 99m Tc–methylene diphosphonate) that is injected intravenously. The radiopharmaceutical binds to the bony matrix of the skeleton over a period of 3 to 6 hours, accumulating at sites of osteoblastic activity. Patients are then scanned from the vertex of the skull to the knees or below to identify sites of abnormal accumulation that suggest metastases. Characteristically, the frequency of metastases to any given bone is proportional to the amount of red marrow that bone contains, and, as a general rule in prostate cancer, metastases occur in the axial skeleton before they occur in the appendicular skeleton. Correlating bone scans with recent planar radiographs or CT is often helpful to distinguish osteophytes or previous trauma from metastases. Nevertheless, the yield of positive findings in bone scans is low in men with early-stage increases in PSA levels. Conventional bone scanning is rarely positive when PSA levels are less than 10.0 ng/mL and remains fairly insensitive when the PSA levels are less than 20.0 ng/mL. In a study at the Mayo Clinic of 306 prostate cancer patients with a serum PSA level of 20 ng/mL or less, Chybowski and colleagues found that only 1 patient, who had a PSA of 18.2 ng/mL, had a positive bone scan. Nevertheless, a conventional radionuclide bone scan is a valuable prognostic indicator in patients with advanced disease because prostate cancer patients with a positive bone scan are known to have a shortened lifespan ( Fig. 2 ).
Additionally, several studies suggest that [ 18 F]sodium fluoride–PET bone scanning is more sensitive and more specific than 99m Tc–methylene diphosphonate bone scanning in identifying osteoblastic metastases in most cancers and may be up to 100% sensitive and specific with 100% positive predictive values and negative predictive values in prostate cancer patients with PSA levels greater than 20 ng/mL. In February 2011, the National Oncologic PET Registry added [ 18 F]sodium fluoride–PET bone scanning as a Medicare-billable diagnostic option for evaluating osseous metastatic lesions for all cancers. The medical literature to date contains no information, however, on the PSA value that correlates with a positive sodium fluoride PET bone scan, and the clinical significance of such a threshold remains to be determined.
Bone scan
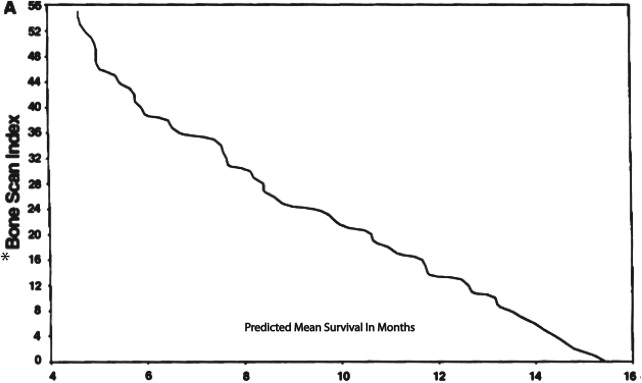
Bone scanning has been used to look for osseous metastases in prostate cancer since the introduction of the modern Anger gamma camera to nuclear medicine in the 1960s. Bone scans are more sensitive for detecting osteoblastic metastasis than are planar radiographs because 30% of the bone matrix must be affected for metastases to be detectable by conventional radiography. Studies dating back to 1976 have shown that at least 62% of patients with a positive bone scan have normal planar radiographs. Bisphosphonates are labeled with radioactive technetium Tc 99m ( 99m Tc) to make a radiopharmaceutical ( 99m Tc–methylene diphosphonate) that is injected intravenously. The radiopharmaceutical binds to the bony matrix of the skeleton over a period of 3 to 6 hours, accumulating at sites of osteoblastic activity. Patients are then scanned from the vertex of the skull to the knees or below to identify sites of abnormal accumulation that suggest metastases. Characteristically, the frequency of metastases to any given bone is proportional to the amount of red marrow that bone contains, and, as a general rule in prostate cancer, metastases occur in the axial skeleton before they occur in the appendicular skeleton. Correlating bone scans with recent planar radiographs or CT is often helpful to distinguish osteophytes or previous trauma from metastases. Nevertheless, the yield of positive findings in bone scans is low in men with early-stage increases in PSA levels. Conventional bone scanning is rarely positive when PSA levels are less than 10.0 ng/mL and remains fairly insensitive when the PSA levels are less than 20.0 ng/mL. In a study at the Mayo Clinic of 306 prostate cancer patients with a serum PSA level of 20 ng/mL or less, Chybowski and colleagues found that only 1 patient, who had a PSA of 18.2 ng/mL, had a positive bone scan. Nevertheless, a conventional radionuclide bone scan is a valuable prognostic indicator in patients with advanced disease because prostate cancer patients with a positive bone scan are known to have a shortened lifespan ( Fig. 2 ).
Additionally, several studies suggest that [ 18 F]sodium fluoride–PET bone scanning is more sensitive and more specific than 99m Tc–methylene diphosphonate bone scanning in identifying osteoblastic metastases in most cancers and may be up to 100% sensitive and specific with 100% positive predictive values and negative predictive values in prostate cancer patients with PSA levels greater than 20 ng/mL. In February 2011, the National Oncologic PET Registry added [ 18 F]sodium fluoride–PET bone scanning as a Medicare-billable diagnostic option for evaluating osseous metastatic lesions for all cancers. The medical literature to date contains no information, however, on the PSA value that correlates with a positive sodium fluoride PET bone scan, and the clinical significance of such a threshold remains to be determined.
Ultrasound
Ultrasound has long been used to detect prostate cancer. In the late 1980s and early 1990s, there was great hope among many of those in the imaging community that, with the development of dedicated ultrasound transducers, TRUS would prove useful for screening. Such was not the case. The findings on TRUS are not sufficiently specific to make it a cost-effective tool for screening the general population. Currently, in addition to guiding biopsies of nodules detected on digital rectal examination for the initial diagnosis of prostate cancer, TRUS is used in looking for recurrence in the prostate bed after radical prostatectomy with subsequent biochemical recurrence heralded by rising PSA levels. Beyond the prostate bed, however, ultrasound lacks sufficient tissue penetration to be of use in looking for pelvic nodes or more distant metastases.
CT
Because CT renders precise anatomic images, it has become the mainstay of advanced imaging in most medical institutions. Although the average CT scanner in most communities today can easily resolve anatomic detail as small as the ossicles of the middle ear, the issue in prostate cancer imaging is specificity not sensitivity. The question is, “What does the finding of a 1-cm lymph node localized in the pelvis of a patient with a rising PSA level suggest?” Does this finding confirm recurrent prostate cancer? Does the finding mean that the node contains metastatic tissue? It may simply be a prominent normal node or perhaps a node enlarged in reaction to infection or inflammation elsewhere in that node’s lymphatic drainage area. Since CT’s inception, radiologists have relied on size criteria—if a node is less than 1 cm, it is benign; if it is more than 1 cm, it is pathologic. Not only is this intuitively illogical because a node must contain a critical mass of metastatic cells before it starts to enlarge but also, statistically, these criteria have been shown to provide less than ideal specificity for diagnosis. A vivid illustration of this is the case of lung cancer. In 2000, even before the advent of the current PET scanners that are coupled to low-dose CT for attenuation correction (PET/CTAC), Pieterman and colleagues showed, in a sample of more than 100 lung cancer patients, that the specificity for detection of mediastinal metastases with FDG-PET was 86% but the specificity for detection with CT was only 66%. This introduces the case for functional molecular imaging over anatomic imaging (discussed later). First MRI is discussed, which is in many ways a hybrid of anatomic and physiologic imaging.
MRI
Because of its unique ability to characterize the content and distribution of water in various tissues of the body and its good anatomic resolution, MRI has shown great promise for the detection of both the primary lesion and metastasis in prostate cancer. It is an excellent technique for localizing the nidus of tumor in the intact prostate gland and can be used in presurgical planning to see whether the tumor has spread beyond the confines of the prostate capsule or has invaded the neurovascular bundle. It has also shown usefulness in anatomically identifying metastatic nodes in the pelvis. Issues of specificity remain unsolved, however. Groundbreaking research in the Netherlands using a ferromagnetic contrast agent with MRI holds promise for improving specificity of diagnosis in finding nodal metastases, but this technique is years away from possible Food and Drug Administration (FDA) approval or Centers for Medicare and Medicaid Services reimbursement certification. Additionally, MRI has been of limited use in finding distant metastases, because, by the nature of the technology, each region of the body needs to be scanned separately, adding to the expense and length of scanning time.
Capromab pendetide SPECT or SPECT/CTAC
First introduced in the late 1990s, capromab is a hamster monoclonal antibody that selectively binds to prostate-specific membrane antigen, a surface biomarker that is present on human prostate cancer cells at various stages but is not present on normal cells. Labeled with 111 In, the antibody is injected into a patient, with SPECT or SPECT/CTAC imaging performed 4 to 6 days later. Although elegant in theory—using an imaging isotope labeled to an antibody that is targeted specifically for prostate cancer cells, in practice the results have been less than stellar. The scans can appear murky, making them difficult to read and the interpretation learning curve for nuclear radiologists is steep. Moreover, correlating capromab scan findings with actual pathologic findings has not always yielded reassuring results. For example, in a prospective, preoperative study with tissue confirmation, Ponsky and colleagues at the Cleveland Clinic found a sensitivity of only 17% and a positive predictive value of only 11% and concluded a high false-positive rate and a low positive predictive value overestimated metastatic lymph node disease. As a result of these difficulties with scan interpretation, many urologists and oncologists have become disillusioned with the examination.
PET/CTAC
Given the limitations of anatomic imaging (described previously), is there any hope for a breakthrough in imaging prostate cancer, especially metastatic or recurrent prostate cancer? The answer may be within the realm of functional molecular imaging, specifically PET coregistered with low-dose CTAC, which gives both metabolic and molecular information on a cellular level coupled with anatomic definition. As discussed previously, FDG-PET has shown a greater specificity compared with anatomic imaging in the staging and management of lung cancer. The differences in sensitivity and specificity for FDG-PET versus CT in the diagnosis, staging, and detecting recurrence in lung cancer have been shown in some series to result in a change in patient management 37% of the time. For colorectal cancer recurrence, change in management is seen in 31% of the patients, with similar advantages seen in lymphoma, melanoma, and breast cancer. Most common cancers—lung, lymphoma, melanoma, colorectal, head and neck, esophageal, transitional cell, and breast—have increased metabolic needs to grow rapidly and proliferate. The basic metabolic substrate for these tumor cells is glucose. To meet the increased need for glucose, these tumor cells have increased glucose transport mechanisms and are thus considered glucose avid. FDG is made by chemically substituting cyclotron-produced radioactive 18 F for a hydroxyl group on the hexose ring of glucose. The resulting compound, FDG, after being phosphorylated, is transported through the cell membrane in the same manner as glucose. The key metabolic difference between glucose and FDG is that FDG is not as easily dephosphorylated and then metabolized by tumor cells as glucose. Therefore, by virtue of its increased transport into the cell and decreased metabolism within the cell, FDG accumulates to a greater extent in tumor cells than surrounding normal tissue. This makes the radioactive signal of the FDG in the tumor cells more conspicuous than the signal from any normal cells that may have also taken up FDG, thus rendering tumors easier to localize than by CT alone. In addition, it allows differentiating metabolically active tumor from scar tissue after therapy.
Unfortunately for prostate cancer patients, prostate cancer cells are not particularly glucose avid, except in more aggressive cell lines, such as those with high Gleason scores. The reason for this is unclear. Some investigators have postulated that because prostate cancer can be more indolent, perhaps it does not have high glucose requirements. This simplistic explanation is contradicted, however, by the fact that another genitourinary tumor, renal cell carcinoma, is highly aggressive yet is also not highly glucose avid. So, the answer must lie in prostate cancer metabolism on a cellular level. The good news is that other compounds that are ubiquitous in the body, acetate and choline, have a unique metabolism in prostate cancer. These compounds are used in the synthesis of the phospholipid bilayer of the cell membrane. In normal prostate cells, acetate is metabolized through the Krebs cycle for energy. With the increased cellular proliferation of prostate cancer, however, acetate is used for cell membrane synthesis. By substituting cyclotron-produced 11 C into the acetate molecule for a nonradioactive carbon 12 atom, [ 11 C]acetate is able to be detected incorporated into the cell membranes of proliferating prostate cancer by means of PET/CTAC.
Data from both the United States and Europe have shown similar results detecting recurrent prostate cancer with either [ 11 C]acetate or [ 18 F]choline. From a practical point of view, [ 18 F]choline is easier to ship from the cyclotron to the PET scan site because 18 F has a 110-minute half-life compared with the 20-minute half-life of 11 C, which generally limits [ 11 C]acetate use to PET scanners within close proximity of a cyclotron. Alternatively, [ 11 C]acetate has little urinary tract excretion whereas [ 18 F]choline has significant urinary excretion that can potentially obscure recurrent tumors in the prostate bed and along the course of the ureters. Finally, in the United States, [ 18 F]choline is not FDA approved, whereas [ 11 C]acetate has been in the United States Pharmacopeia for more than 20 years with a great patient safety record in cardiac PET imaging. Since the first article in the medical literature describing the utility of [ 11 C]acetate in imaging prostate cancer by Oyama and colleagues in 2002, there have been more than 50 refereed journal articles in the world literature describing the application of [ 11 C]acetate and [ 18 F]choline-PET in prostate cancer. Since 2002, however, most of the [ 11 C]acetate and [ 18 F]choline clinical PET prostate studies have been performed in Europe, because difficulties in getting FDA approval and CMS reimbursement are not an issue in Europe as they have been in the United States.
In 1998, Haseman published a comparison of capromab SPECT versus FDG-PET and found capromab superior. In 2003, Oyama and colleagues compared [ 11 C]acetate-PET to FDG-PET in 46 patients and found [ 11 C]acetate to have a putative sensitivity of 59% versus 17% for FDG-PET. The third leg of the metaphorical triangle would be to compare capromab SPECT to [ 11 C]acetate-PET, but to date there have been no published data comparing the 2 studies. However, between February 22, 2007 and February 21, 2008, in the Department of Radiology, Division of Nuclear Medicine at the University of Kansas Hospital, Kansas City, Kansas, the authors conducted a pilot study prospectively comparing capromab SPECT to [ 11 C]acetate-PET in each of 20 men who had undergone radical prostatectomy for primary prostate cancer and experienced biochemical recurrence, defined as a rise in PSA postprostatectomy from a baseline of 0.0 ng/mL to 0.2 ng/mL or greater. Using 3 nuclear medicine subspecialty trained board certified radiologists who read all the studies in a blinded manner, the results showed that for early recurrence—an average lymph node size of 1.03 cm and a mean PSA value of 1.0—there was a putative sensitivity of 85% for [ 11 C]acetate-PET/CTAC versus a 30% putative sensitivity for capromab SPECT.
Based on these results and findings in the world literature corroborating the high sensitivity of [ 11 C]acetate-PET/CTAC in detecting metastatic prostate cancer, the authors embarked on a program offering [ 11 C]acetate-PET scans to physician-referred patients with biochemically recurrent prostate cancer—currently defined as patients who have undergone a radical prostatectomy and who achieved a postoperative PSA value of 0.0 ng/mL that subsequently rose to greater than 0.4 ng/mL or for patients who have received definitive external beam radiotherapy or brachytherapy, with recurrence defined as a PSA rise of greater than 2.0 ng/mL above the post-treatment nadir. Since 2007, the authors have performed more than 200 clinical [ 11 C]acetate-PET scans at the University of Kansas Hospital, with encouraging clinical results based on outcomes. The following 3 cases serve as illustrations of the potential of functional molecular imaging.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




