Fig. 9.1
The multistage process of prostate cancer development and tumor progression. PCa prostate cancer
The different PCa tumors have varying growth rates and malignant potential for causing death. Screening for PCa should ideally target only tumors that would cause clinically important disease, but currently availably prognostic markers can only distinguish a small number of men having excellent prognosis [8]. However, they cannot say anything about the prognosis of those in the middle category (Gleason grades 3 + 4 vs. 4 + 3), which includes most of the men with PCa [9, 10]. Treatment decision is usually based on PSA levels and histopathological findings in biopsies, i.e., Gleason score.
PSA measurements have significant influence on diagnosis, treatment strategies and follow-up of PCa patients; however the specificity of total serum PSA is limited to treatment strategies especially for patients with low PSA serum levels (less than 4.0 ng/ml) [11–16] The specificity of PSA screening is lower among men with large prostate glands, including older men with benign prostatic hyperplasia (BPH) [17] The discrepancy between PCa diagnoses and PCa deaths indicates that probably most cancers detected by screening are clinically unimportant. Appropriate target that will detect the cancers causing clinical symptoms and death (i.e., Gleason score ≥7) has yet to be defined.
9.2 Histological Diagnoses of Prostate Cancer
Specimen Preparation
Radical prostatectomy (RP) specimens are usually transported in buffered 4 % formaline saline, occasionally, fresh tissue is harvested in the laboratories for research reasons. However, is has been pointed out that the specimens should be placed in fixative as soon as possible, because of alteration in protein, DNA or RNA confirmation that must be satisfactory for the preservation of microscopic and IHC features [18]. The histological diagnosis of PCa is based on formalin fixed and paraffin embedded tissue. The initial diagnosis is based on needle biopsies, usually four or more biopsies from each patient. Prognosis after RP relies, in part, on accurate characterization of a number of histological features, including the status of the surgical margin, extra prostatic tumor growth, Gleason grade, tumor volume and whether tumor extends into the seminal vesicles and/or is metastatic to regional lymph nodes. The 2009 International Society of Urological Pathology Consensus Conference in Boston made recommendations regarding the standardization of pathology reporting of RP specimens [19, 20]. The use of whole mounts of sections from RP specimens has the advantage of displaying the whole architecture of the prostate and the location of tumor areas. Of most importance is assessment of free resection margins, therefore painting the surfaces gives more precise information about the margins [19, 20]. Separate histological examination of both apex and base should be prepared by cutting sagittal sections of the conus (Fig. 9.2) [19]. Histological examination of tumor tissues in prostatic apex and base predicts outcome [21].


Fig. 9.2
Whole-mount tissue section of prostate (center) with sagittal sectioning of the base (top) and apex (below). For orientation and with regard to surgical reception margins, the prostate is inked blue to the right, green to the left and black at the posterior surgical margin
Positive lymphatic involvement is generally considered as a poor prognostic indicator and has impact on treatment options [22]. Identifying lymphatic involvement requires accurate nodal staging. Some laboratories report enhanced retrieval of lymph nodes using Glacial acetic acid, ethanol, distilled water, and formaldehyde (GEWF), compared to formalin fixed specimens [23].
9.3 Microscopic Diagnoses
The Gleason Grading System
The Gleason grading system is so far the most commonly used system for histological characterization of prostate cancer, and it’s the most powerful predictor of outcome for PCa patients [23]. Gleason grading depends solely on architectural patterns of the tumor. The grade is defined as the sum of the two most common grade patterns and reported as the Gleason score. Both the primary (predominant) and the secondary (second most prevalent) architectural patterns are identified and assigned a number from 1 to 5, were 1 being the most differentiated and 5 the least differentiated pattern [23]. Since the introduction of the Gleason grading system more than four decades ago many aspects of prostate cancer have changed, including PSA testing, transrectal ultrasound guided prostate needle biopsies with sampling and immunohistochemistry for identification of basal cells. These techniques have changed the classification of prostate cancer and led to identification of new prostate cancer variants. Gleason grading system was updated in 2005 by experts in urological pathology at a consensus conference held by the International Society of Urological Pathology (ISUP). This has resulted in a more accurate grading system in both needle biopsies and RPs [24–27]. This modified system has also led to a better correlation between pathological stage, rate of positive margins, and biochemical recurrence with Gleason scores. Still, Gleason score is the only independent predictor of metastatic disease [28].. The main differences between the original system and the 2005 ISUP Modified Gleason grading system are summarized in Table 9.1 [24, 26, 27, 29].
Table 9.1
Gleason score comparison
Original Gleason system | 2005 ISUP modified Gleason system |
|---|---|
A diagnosis of GS <4 possible NB | GS of NB specimens <4 rarely, if ever made |
Cribriform glands with rounded and smooth contours as well as with an irregular outer border are diagnosed as Gleason pattern 3 | Most cribriform patterns with only rare cribriform lesions would satisfy the diagnostic criteria for cribriform pattern 3 |
The same GS is used for NB and RP specimens | Different GS used for NB and RP specimens |
High-grade tumor of small quantity (<5 %) on NB should be excluded based on GS (5% threshold rule) | Different GS used for NB and RP specimens |
High-grade tumor of small quantity (< 5%) on NB should be excluded based on GS (<5 % threshold rule) | High-grade tumor of any quantity on NB should be include within the GS |
Tumors on NB should be graded by listing the primary and secondary patterns, i.e., excluding tertiary patterns | For the tertiary pattern on NB specimens, both the primary pattern and the highest grade should be recorded |
The GS of RP specimens should be assigned based on the primary and secondary patterns | For RP specimens, the pathologist should assign the GS based on the primary and secondary patterns; a comment should be added on the tertiary pattern |
Separate or overall scoring to assess all grades of NB specimens | When NB specimens show different grads in separate cores, individual GS should be assigned to these cores (separate scoring) |
The grade of the largest portion should be assigned, even if the second largest portion is of higher grade | When RP specimens show different grades in separate tumor nodules, a separate GS should be assigned to each of the dominant tumor nodules |
PTNM-Classification
The pTNM-classification is currently under revision. Within category pT2 PCa, a wide variation in tumor extent may be seen, which varies from single microscopic lesions to large volume multifocal tumors, often involving both sides of the prostate. This heterogeneity of tumor volume of pT2 prostate cancers and the potential impact on prognostic assessment has resulted in attempts to subcategorize organ-confined tumors. In TNM 2002, pT2 was substaged in pT2a (tumor involves one half of one lobe or less), pT2b (tumor involves more than half of one lobe, but not both lobes), and pT2c (tumor involves both lobes). In the TNM 2010 staging, the pathological substaging of pT2 prostate cancers has been retained, although the prognostic value of this has been questioned.
9.4 Immunohistochemistry (IHC)
IHC is widely used for diagnosing PCa in difficult cases. Awareness of the many pitfalls in prostate IHC is essential to avoid serious misdiagnosis i.e., identifying IHC staining as positive or negative. However, in the majority of cases haematoxylin-Eosin stained sections are sufficient for diagnosing cancer. The IHC diagnosis of PCa largely depends on panels of markers because no absolutely specific and sensitive marker has yet been discovered. Such panels usually include at least one basal cell-specific marker; high molecular-weight cytokeratin (HMWCK), or p63, and the prostate cancer-specific marker; alpha-methyl-CoA-Racemase (AMACR, antibody clone P504S). HMWCK or P63 is expressed in almost all normal basal cells of prostate with continuous intact circumferential immunostaining in most glands. However, HMWCK positivity is typically discontinuous in a variety of benign lesions such as post atrophic hyperplasia and atypical adenomatous hyperplasia, whereas in PCa with the loss of basal cells, these markers are negative. Almost all PCa of low and intermediate grade are negative for p63, while normal or hyperplastic prostatic gland, show strong and diffuse p63 expression. AMACR is usually overexpressed in PCa with strong coarsely granular staining and more accentuated along the luminal border. However, strong expression of AMACR can also be found in high grade prostatic intraepithelial neoplasia (HGPIN) lesions, but weak expression may be found in benign glands, and should not be interpreted as positive staining.
9.5 Role of Genetic Markers in Prostate Cancer
The huge advances in genotyping and gene sequencing technologies has potential to aid disease screening, improve prognostic discrimination and prediction of response to treatment.
PSA
Early detection of PCa through PSA screening has resulted in detection of men with PCa at earlier stages and with lower Gleason grade, but has also contributed to concerns about overdiagnosis and overtreatment of clinically insignificant disease. As a single test serum PSA has limitations, because some men with very low serum levels of PSA (<4.0 ng/ml) will develop prostate cancer (~15 %) [13], and men with benign conditions including BPH, prostatitis and medical intervention frequently elevate serum PSA. This makes serum PSA more sensitive than specific for PCa. However serum PSA is of great value in disease monitoring of existing cancer and for identification of recurrent disease after treatment. Among men undergoing radical prostatectomy, the persistence of undetectable serum levels of PSA can be used to confirm absence of recurrent disease.
Tumor Specific Autoantibodies, AMACR
In order to react against a tumor, the immune system must have antigens that are recognized as foreign. A number of alterations in gene expression occur in cells during tumorigenesis. Tumorigenesis may lead to expression of new antigens or alteration in existing antigens that are found in normal cells. These antigens may include membrane receptors, regulators of cell cycle and apoptosis, or molecules involved in signal transduction pathways. In PCa, AMACR, is a mitochondrial and peroxisomal enzyme that is involved in bile acid biosynthesis and beta-oxidation of branched-chain fatty acids. AMACR is overexpressed in PCa epithelium, making it a specific marker for cancer cells within the prostate gland. Furthermore, overexpression of AMACR is found in premalignant lesions like HGPIN, may increase PIN, may increase the risk of PCa. I addition, genome-wide scans for linkage in hereditary PCa families suggest that the chromosomal region for AMACR (5p13) is the location of a PCa susceptibility gene. Also, experimental studies have shown that loss of AMACR expression slows the growth of some PCa cell lines. As a biomarker AMACR is of important value in PCa.
9.6 Bladder Cancer
Histologically, most urinary bladder cancers (UC) are transitional cell carcinomas (TCC), but approximately 10 % includes squamous cell carcinoma and adenocarcinomas, both rising from the inner lining of the bladder probably because of chronic irritation and inflammation. A minor portion is small cell carcinoma and sarcoma. UC is one of the most common malignancies among men in Western countries (ratio worldwide is about 3.5:1 compared to women) [13]. Except for Japan, the highest incidence is observed in developed countries. Northern Africa and western Asia is also included among high risk areas, the latter is probably related to presence of urinary schistosomiasis (well-known to be associated with high risk of bladder cancer). Cigarette smoking is the predominant risk factor, followed by a small group with occupational exposure to aromatic amines and other industrial chemicals. Also a genetic predisposition is a considerable factor [34, 35]. The clinical behavior of the disease is heterogeneous, ranging from tumors with low malignant potential to highly malignant muscle-infiltrating tumors. Despite refined histology based classification systems, it is difficult to predict individual prognosis or response to therapy. For instance, one third of patients with T1 tumors remain recurrence-free after BCG treatment, while one third die from the disease. Classification schemes for bladder tumors, especially for TCC, which represents the vast majority of cases, have evolved over the past decades, and will continue to change as information regarding genomics and proteomics accumulates. Current prognostic parameters such as grade, stage, multifocality of carcinomas, and lymph node status cannot with certainty predict the long term outcome of bladder cancer.
Staging of bladder cancer
The prognosis and treatment decisions are mainly based on pathological criteria, and proper staging is highly dependent on good quality biopsies. Approximately 70 % of these tumors are papillary and confined to the urothelial mucosa (stage Ta) or to lamina propria (T1), whereas, the remaining invade the muscle (T2), the perivesical fat (T3) or surrounding organs (T4) (Fig. 9.3). Favorable prognostic factors for superficial TCC at stage Ta, compared to stage T1 are: low grade and no dysplasia, whereas high grade, tumor multiplicity, tumor size greater than 5 cm, and vascular invasion increase the risk for tumor progression.
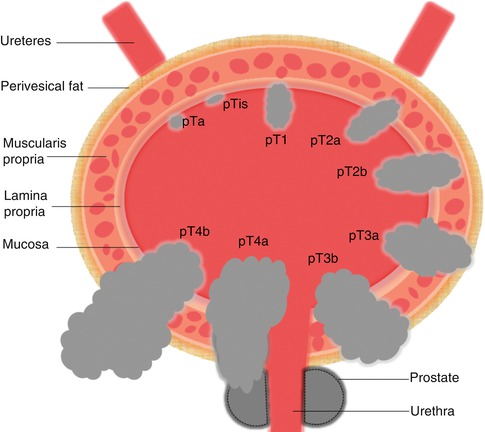

Fig. 9.3
Bladder cancer staging
Histological Diagnoses of Bladder Cancer
Specimen Preparation
Among the established methods for detecting and confirming bladder cancer are bimanual palpation, cystoscopy with or without random or selected-site biopsies and urinary cytology.
In clinical routine, mainly formalin-fixed and paraffin-embedded tissue has been preserved, and by using light microscope, the histologic diagnoses are done according to World Health Organization (WHO)/International Society of Urological Pathology (ISUP) [36].
Urinary cytopathology is especially useful for detection and monitoring of patients with epithelial tumors, but this approach is best applied to high-grade tumors, where nearly all of the features are detectable in these specimens. This means that nearly all aggressive tumors can be detected in adequately sampled bladders. This method is less useful for the detection and monitoring of very low-grade tumors, because most of these tumors lack feature of malignancy [36].
Microscopic Diagnoses and Classification
TCC (urothelial carcinoma) can rise anywhere in the bladder. They are defined according to their histologic characteristics and so far, histologic examination is the most specific method for assessing these lesions [37]. The pattern of growth may be exophytic or endophytic or a combination of both. When histologically exophytic, the tumor may adopt a papillary configuration with a fibrovascular core, or a solid nodular appearance. The latter may result in clusters of tumor cells in the lamina propria. Stromal invasion of TCC proceed in two stages: invasion of the lamina propria and invasion of the muscle layer. The detection of muscle invasion is of great consequence, because of the influence on therapy and prognosis. Histological diagnose of early bladder cancer (superficial TCC) or non-muscle invasive bladder cancer, which includes papilloma (benign lesions), papillary urothelial neoplasms with low malignant potential (PUNLMP), Low grade papillary carcinomas CIS (TIS/CIS), is virtually never confused with high grade carcinomas. PUNLMP have many features in common with normal epithelium and the histologic feature of the cells on the stalks is preserved and the nuclear feature are only slightly abnormal [38]. Lacking significant nuclear pleomorphic appearance, PUNLMP cells are difficult to recognize in urinary samples. However, this entity is considered as neoplastic because they tend to recur in the same site, and if left untreated, they can grow and dedifferentiate into aggressive cancers. Urothelial carcinoma, low grade, is a predominantly papillary urothelial neoplasm which resembling PUNLMP features. Histologically, the superficial layer is preserved, and the cells are uniform in size and evenly distributed. The orientation of the nuclei are often normal, but are rounded and slightly pleomorphic. There distinction of PUNLMP from low grade carcinomas may be difficult. However, the cells of low grade carcinoma are smaller and more densely arranged on the fibrovascular stalks, than the cells of PUNLMP, and at high magnification, there is a slight degree of nuclear pleomorphism and the nuclear chromatin is finely granular and evenly distributed. Immunohistochemical analyses are unlikely to be helpful in the differential diagnosis. The tumor cells are typically uniform, but densely packed in tissue sections. The tumor cells have indistinct borders, little or no cytoplasm, and the nuclear are pleomorphic, and the nuclear borders are irregular with coarse and uneven chromatin structure. Almost all invasive urothelial neoplasms are high grade, at least there is a focus of invasion, whether the invasion is confined to the lamina propria or muscularis propria. Mitoses are common and may be abnormal. Differential diagnostic problems are not a large problem, but may raise when high TCC involve prostatic duct or when prostatic carcinomas have invaded the bladder base. However, there are histological differences between high grade TCC and prostate cancer cells. Of importance is to diagnose patients whom superficial tumors are less differentiated, large, multiple, or associated with CIS in other areas of the bladder mucosa. These patients are at great risk for recurrence and the development of invasive cancers. IHC is overall of limited value when reactions for cytokeratins (7 and 20), carcinoembryonic antigens (CEA)125, 34β[beta]E12, and thrombomodulin are not specific. However, cytokeratins (CK20, CK7), tumor suppressor gene (p53) and the proliferation marker, Ki67 is widely used, because the majority of urothelial carcinomas are CK7+/CK20/CEA125-. The expression of CK20 is restricted to superficial ‘umbrella’ cells and occasional in the intermediate cells in benign and reactive urothelium, and also in severe inflammation. In dysplasia and CIS there is usually a complete or at least focal loss of this cellular reaction in all layers of the urothelium [39]. CK20/CK7 positivity may be useful when both were positive, supporting the diagnose of urothelial cancer. However, if only one marker was positive, or both negative, these markers have limited usefulness for distinguish these carcinomas. Expression of p53 has also been associated with an adverse prognosis for patients with invasive bladder cancer. Retrospective studies have shown that presence of nuclear p53 is an independent predictor for recurrence among patients with T-1-T3 tumors [40–43].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








