Fig. 9.1
Kaplan–Meier renal survival from renal biopsy (a) and disease onset (b) in 1,155 patients with IgAN. ESRD end-stage of renal disease
For patients with proteinuria >1.0 g/day at biopsy, the 10-, 15- and 20-year cumulative renal survival rates from renal biopsy were 71 %, 63 %, and 54 %, respectively, and the 10-, 15- and 20-year cumulative renal survival rates from renal disease onset were 83 %, 72 %, and 67 %, respectively (Fig. 9.1).
The 20-year renal survival rate after renal biopsy in this cohort of patients (64 %) was highly similar to those of patients from Japan and Korea. A study of 1,012 patients in Japan reported that the 20-year cumulative survival rate from renal biopsy to ESRD was 66.6 % [6], and a study of 1,364 patients in Korea reported that the 20-year cumulative renal survival rate was 66.9 % [7]. The variability in renal outcome of IgAN in different studies may account for the difference in indications of kidney biopsy and health screening practice to some extent. Patients with selective biopsy indications would have lower renal survival rates. In this study, the indication of renal biopsy was relatively less selective, as suggested by the lower incidence of renal insufficiency (21.3 %), proteinuria >1.0 g/day (43.4 %), and hypertension (29.7 %) at the time of biopsy.
9.2.1 Long-Term Renal Outcome in IgA Nephropathy Patients with Recurrent Macro-Hematuria
Macro- and microscopic hematuria are frequent in patients with IgAN. However, the prognostic value of hematuria is highly complex in patients with IgAN. The persistent microscopic hematuria is associated with an increased incidence of renal failure, whereas episodic recurrent macro-hematuria (MH) is associated with relative benign renal outcomes [1, 8, 9].
Patients with recurrent hematuria (MH) (RMH, episodes of MH≥2 times, episode intervals >1 months) are a distinct subtype of IgAN [5]. Of the 1,155 patients with IgAN, 158 (14 %) of them presented with a history of RMH at biopsy. Compared with patients without a history of MH (NMH), patients with RMH were younger and more likely to be female and had lower proteinuria, a lower incidence of hypertension, a higher eGFR, a lower level of mesangial hypercellularity and segmental glomerulosclerosis, and a lower level of tubular atrophy. More importantly, as shown in Fig. 9.2, the long-term renal prognosis in RMH patients is dramatically better than that in NMH patients. The renal prognosis of patients with RMH was also better than those with isolated MH (IMH, one episode of MH) [10]. During a median follow-up of 7.9 years, the 10- and 20-year cumulative renal survival rates after biopsy, as calculated by K–M methods, were 91 % and 91 % in the RMH group, 89 % and 64 % in the IMH group, and 79 % and 57 % in the NMH group, respectively.
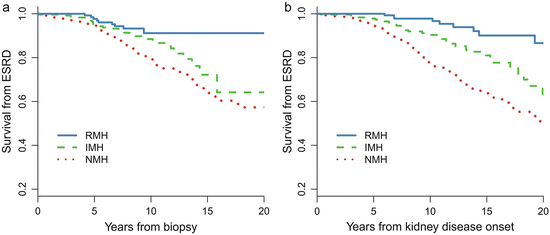

Fig. 9.2
Kaplan–Meier renal survival curve in IgAN patients with different MH patterns. NMH no history of macro-hematuria, IMH isolated onset of macro-hematuria, and RMH recurrent onset of macro-hematuria
Why patients with recurrent episodes of MH had relative benign renal outcomes remains unknown. It has been hypothesized that a kidney injury mechanism, mainly triggered by an upper respiratory infection, is more evident but discontinuous in patients with RMH, whereas in those without RMH, the injury mechanism is less potent but persists and could induce more severe and progressive kidney damage over time [1]. Moreover, little is known about the molecular mechanisms that cause episodic MH in IgAN [11].
9.2.2 Long-Term Renal Outcome in IgA Nephropathy Patients with Minimal Change Disease
There is another special entity of IgAN called IgAN with minimal change disease (MCD-IgAN), which was first reported in 1986 [12]. Those patients clinically present with nephrotic syndrome and a lack or mild degree of microscopic hematuria and have good response to corticosteroid therapy; meanwhile, pathologically, they have mild glomerular lesions under light microscopy, diffuse IgA-dominant depositions in mesangial region, and diffuse effacement of podocyte foot process and mesangial electron-dense deposition under electron microscopy. MCD-IgAN accounted for 2.6 % of the total biopsy-proven IgAN in our center. However, the clinicopathological characteristics and long-term renal outcome of MCD-IgAN in large cohorts have not yet been well described.
A retrospective review of 247 cases of biopsy-proven MCD-IgAN from the Glomerulonephritis Registry in Jinling Hospital was performed to characterize its clinicopathological features, treatment response, and long-term prognosis. In this cohort of patients, there was a male predominance (67.7 %), with an average age of 27.1 ± 11.0 years at biopsy. Pathological data revealed that 34 patients (13.7 %) had moderate to severe acute tubulointerstitial lesions and that 11 cases (4.5 %) had mild chronic tubulointerstitial lesions. After an 8-week course of corticosteroid therapy, a complete remission of proteinuria was observed in 216 patients (87.4 %), partial remission was observed in 21 patients (8.5 %), and no remission was observed in 10 patients (4.0 %). No patients with MCD-IgAN progressed to ESRD. MCD-IgAN patients had significantly better renal outcome compared with the cohort of 1,155 IgAN patients reported previously (Fig. 9.3).
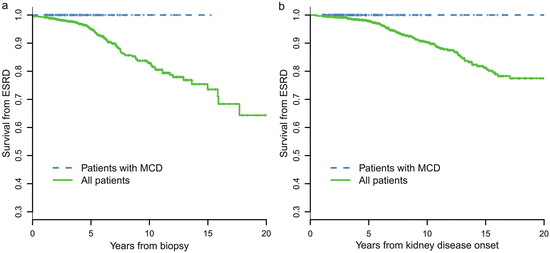

Fig. 9.3
Comparison of cumulative probabilities of renal survival between MCD-IgAN and non-MCD-IgAN patients
9.3 Prognostic Indicators in IgA Nephropathy
9.3.1 Clinical Prognostic Indicators
It is important to predict which patients are at high risk of progression and should be treated. Using the 1,155-patient follow-up data, a univariate Cox regression model found that eGFR, hypertension, proteinuria, hypoproteinemia, hyperuricemia, hypercholesterolemia, hypertriglyceridemia, and body mass index (BMI) were associated with a combined renal outcome (ESRD or 50 % drop in eGFR) during follow-up. Multivariate Cox model confirmed that eGFR, proteinuria, hypoproteinemia, hypertension at presentation, and hyperuricemia were the five most important clinical predictors independently associated with renal outcome at the time of biopsy.
9.3.2 Prognostic Indicators During Follow-Up
The effects of clinical parameters during follow-up on the outcome were also examined in the 1,155 patients with IgAN. The summary measures (time-weighted averages) for proteinuria, blood pressure (MAP), and microscopic hematuria were calculated for each patient from the area under the curve of serial measurements standardized by the duration of follow-up. For instance, the time-average MAP (TA-MAP) was defined as the ratio of the area under the curve of MAP during follow-up to the duration of follow-up. The time-average blood pressure (TA-BP) was calculated with the same method as TA-MAP. Similarly, for each patient, the time-average proteinuria (TA-P) represents the area under the curve of proteinuria during follow-up divided by the years of total follow-up. As microscopic hematuria was highly skewed during the follow-up, logarithmic transformation was used to stabilize variance of the microscopic hematuria. After log transformation of microscopic hematuria, we also calculated the time-average microscopic hematuria (TA-RBC) in a similar manner. The TA-P, TA-RBC, and TA-MAP were all associated with renal outcome in both univariate and multivariate Cox models [5].
The predictive value of proteinuria and blood pressure is well defined in previous studies, whereas the predictive value of the extent of microscopic hematuria, measured as the count of urinary erythrocytes, remains uncertain; microscopic hematuria is extremely variable over time in the same patient. We did not find an association between renal outcome and the extent of microscopic hematuria at the time of renal biopsy. However, TA-RBC was associated with renal outcome when TA-RBC was used to quantify the extent of microscopic hematuria during follow-up. The multivariate Cox model showed that the risk for a combined event increased by 210 % for every ten-fold increase in microscopic hematuria.
9.3.2.1 Target Levels of Proteinuria During the Treatment of IgA Nephropathy
Proteinuria has been proven to be the most important predictor of renal failure in IgAN. However, the threshold of proteinuria above which the risk develops, and the goal of a safe proteinuria level during follow-up, remains the subject of debate. Evidence remains lacking that the long-term renal outcomes differ between patients with a proteinuria at 0.5–1.0 g/day and those at <0.5 g/day. We used the TA-P to quantify the mean exposure of proteinuria during the follow-up in patients with IgAN, and a ROC of TA-P was drawn to determine the optimal cutoffs predicting a worse renal outcome (ESRD or 50 % reduction in eGFR) [5]. The area under ROC (AUC) was 0.9 for TA-P, indicating that TA-P had high diagnostic accuracy for an unfavorable renal outcome. The optimal cutoff of TA-P was 0.97 g/day (sensitivity 83 %, specificity 84 %) in this cohort, which was approximated to 1.0 g/day to facilitate clinical practice, indicating that the relationship between TA-P and renal outcome is dramatically altered down to levels as low as 1.0 g/day. Patients with TA-P >1.0 g/day were associated with a 14-fold higher risk of the combined events than those with TA-P <1.0 g/day (p < 0.001) [2].
In addition, we found that patients with TA-P <0.5 g/day had a significantly more favorable renal outcome than those with TA-P between 0.5 and 1.0 g/day. As shown in Fig. 9.4, the cumulative renal survival from ESRD, as well as from the combined renal events in patients with TA-P 0.5–1.0, was significantly lower than those with TA-P <0.5 g/day. The multivariate Cox analysis, adjusted with TA-MAP, TA-RBC, and eGFR at biopsy, showed that patients with TA-P >1.0 g/day and patients with TA-P 0.5–0.1.0 g/day were associated with 76.5-fold (p < 0.001) and 13.2-fold (p = 0.01), respectively, increased risks of ESRD compared with those with TA-P <0.5 g/day (Fig. 9.5a). The multivariate Cox analysis also showed that patients with TA-P >1.0 g/day and patients with TA-P 0.5–0.1.0 g/ day were associated with a 46.5-fold (p < 0.001) and 9.1-fold (p < 0.01), respectively, increased risk of ESRD or 50 % drop in eGFR during follow-up than those with TA-P <0.5 g/day (Fig. 9.5b).
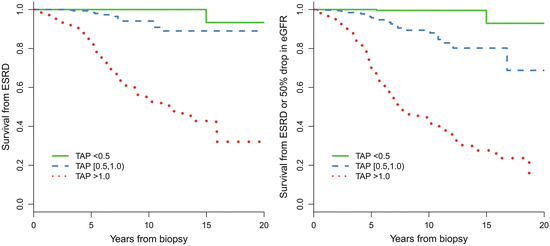
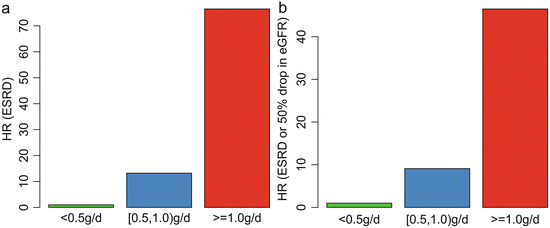

Fig. 9.4
Predictive value in renal survival of time-averaged proteinuria

Fig. 9.5
Associations between the TA-P and hazard ratio of ESRD (a) and the combined renal outcome (b). The results were adjusted with TA-MAP, TA-RBC, and eGFR at biopsy in the multivariate Cox analysis
These results suggest that the basic goal of anti-proteinuric therapy for Chinese patients is to lower proteinuria to <1.0 g/day, whereas the optimal goal is to lower proteinuria to <0.5 g/day.
9.3.3 Pathological Prognostic Indicators
9.3.3.1 Validation of the Oxford Classification in China
The Oxford classification identified four definitive histologic lesions with high reproducibility, low collinearity, and independence of clinical factors. These lesions were mesangial hypercellularity (M), endocapillary proliferation (E), segmental sclerosis or adhesion (S), and tubular atrophy/interstitial fibrosis (T) [13, 14]. Several validation studies were performed after the Oxford classification of IgAN was published and were reviewed by Roberts [15].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







