2MedStar Georgetown University Hospital, Washington, DC, USA
3 The University of Texas MD Anderson Cancer Center, Houston, TX, USA
- Primary hepatic or hepatocellular cancer (HCC) is among the most common tumors worldwide, and its incidence is rising in many countries, including the United States.
- In the majority of cases, HCC occurs against a background of chronic liver disease or cirrhosis, providing a well-defined “at-risk” population for whom surveillance strategies may be employed. Diagnosis of HCC depends primarily on imaging studies. However, biopsy and serum biomarkers are also utilized.
- Although outcome is poor for patients with advanced HCC, those diagnosed at an early stage can be managed effectively with a variety of curative options. Treatment is dependent on a range of factors, particularly on the size and stage of the tumor, as well as hepatic function.
- Novel biomarkers and molecular therapy aimed at earlier diagnosis and more effective treatment therapy carries significant potential for more effective management of HCC.
NCCN Clinical Practice Guidelines in Oncology
Child-Pugh Score Calculator
- Only 15% of hepatocellular carcinoma (HCC) patients are potentially amenable to curative therapies at the time of presentation. Hence, screening at-risk populations for the development of HCC is essential.
- Alpha-fetoprotein as a serum marker for HCC lacks adequate sensitivity and specificity for effective surveillance and diagnosis of HCC. Therefore, screening needs to be based on imaging studies such as abdominal ultrasound at 6-month intervals.
- A single dynamic imaging technique (i.e., four-phase multidetector computed tomography or dynamic contrast-enhanced MRI) is sufficient for diagnosing HCC of more than 1 cm in size if arterial enhancement is observed followed by “washout” in the venous-delayed phase.
- These characteristic, radiologic patterns can be missing in the following situations and should prompt consideration of a targeted liver biopsy:
- HCC of less than 2 cm can be hypovascular and present hypointense on the arterial as well as portal venous phase. In this situation, a liver biopsy will aid in distinguishing between HCC and a dysplastic nodule.
- Cholangiocarcinomas can present as false positive for HCC. Therefore, a targeted liver biopsy of such lesions is required in cases of discrepancies between imaging studies.
- HCC of less than 2 cm can be hypovascular and present hypointense on the arterial as well as portal venous phase. In this situation, a liver biopsy will aid in distinguishing between HCC and a dysplastic nodule.
- 5-year survival rates after orthotopic liver transplantation for HCC are 70%. In contrast, 5-year survival rates for intrahepatic cholangiocarcinomas outside of carefully conducted treatment protocols are less than 20%. Therefore, certainty of the diagnosis of HCC is highly important.
Epidemiology
Primary hepatic cancer is the fifth most common cancer worldwide and the third leading cause of cancer mortality in the United States, after lung and stomach cancer.1 An estimated 24,120 new cases from liver and intrahepatic bile duct cancer in the United States are expected to occur during 2010, resulting in approximately 18,910 deaths. The incidence of hepatocellular carcinoma (HCC) has been steadily increasing since the early 1980s, with approximately 80% of cases due to an underlying chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection.2 HCC is second only to thyroid cancer with regard to the increase in rates of incidence from 1994 to 2003.3
The incidence of HCC varies widely according to geographic location with differences in distribution likely due to variations in exposure to the hepatitis viruses. High incidence regions of HCC (>20/100,000) occur in sub-Saharan Africa and eastern Asia, with China alone accounting for more than 50% of new cases.1 North and South America, most of Europe, Oceania, and parts of the Middle East are areas of low incidence (<5/100,000).
HCC is the fastest growing cause of cancer-related death in men in the United States.4 Both incidence and mortality rates are twice as high in men as in women (incidence rates of 5.0 and 1.3 per 100,000 population, respectively).4 HCV is the leading contributor to HCC in the United States and other Western nations.5,6 Of the estimated 2.7–3.9 million people in the United States who are chronic carriers of hepatitis C, approximately 20% will develop cirrhosis and 5% will ultimately die from HCC.5 High infection rates with hepatitis C between 1960 and 1990 and the lag time of 20–30 years between virus acquisition and development of cirrhosis and carcinoma have been largely responsible for the tripling of HCC incidence in the United States between 1975 and 2005.7 HCC disproportionately affects minorities—the age-adjusted incidence rates are higher in those of Asian descent (8/100,000 population), Hispanics (6/100,000 population), and African Americans (5/100,000 population) as compared with Caucasians (2.5/100,000 population).8
Recently, the combination of insulin resistance, hypertension, dyslipidemia, and obesity, termed “metabolic syndrome,” has been recognized as a cause of nonalcoholic fatty liver disease (NAFLD). There is increasing evidence that the risk of developing HCC in NAFLD cirrhosis is between 18% and 27%, which is greater than the risk of developing HCC in HCV-related cirrhosis.9 Hemochromatosis is also a significant risk factor for HCC, with an increased relative risk 200 times that of the normal population.
Diagnosis
There are a number of tests utilized in the diagnosis of HCC including radiological studies, histopathology, and analysis of serum biomarkers.10 The American Association for the Study of Liver Diseases (AASLD) guidelines on the management of HCC provide a framework for the approach to diagnosis.11
Clinical features
HCC is typically asymptomatic and most signs and symptoms of the disease relate to the patient’s chronic liver disease. The most commonly reported symptoms are nonspecific and include phrenic irritation causing vague upper abdominal pain or right shoulder discomfort. Patients may also report fatigue, weight loss, jaundice, early satiety, anorexia, and fever, although these symptoms are more often found in advanced lesions. On physical examination, patients with HCC may have hepatomegaly, ascites, splenomegaly, jaundice, a palpable upper abdominal mass, hepatic bruit, or fever.12 A paraneoplastic syndrome may also develop in patients with HCC, and such patients may present with hypercalcemia, hypoglycemia, diarrhea, erythrocytosis, hypercholesterolemia, gynecomastia, and virilization.13
Imaging studies
HCC is a highly vascular tumor, receiving the majority of its blood supply from branches of the hepatic artery, as opposed to the liver parenchyma that receives 70% of its supply from the portal vein.14 This “arterialization” of the vascular supply to the tumor accounts for its classic imaging hallmark: enhancement in the arterial phase and washout of contrast media in the portal venous phase.15 Imaging modalities used in diagnosis include ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and angiography.16 Contrast-enhanced studies allow for the diagnosis of HCC without necessitating biopsy; four-phase helical CT and multiphase dynamic contrast-enhanced MRI are the most reliable imaging tests for HCC.17
Ultrasound is the preferred test in screening for HCC. However, imaging quality is dependent both on the operator and patient body habitus. Neoplastic lesions less than 3 cm in size are typically hypoechoic, well circumscribed, and homogenous. As tumor size exceeds 3 cm, the appearance on ultrasound is more heterogenous, isoechoic, or hyperechoic, and central hypoechoic regions representing fibrous septae may be observed.18 Ultrasound can also reveal vascular patency or intrahepatic thrombosis, and color Doppler ultrasound can provide an estimate of mean velocity blood flow of the hepatic vessels. Contrast-enhanced ultrasonography is not widely utilized in the United States, but has been shown in several studies to have superior accuracy to standard ultrasound.
Four-phase helical CT consists of unenhanced, hepatic arterial, portal venous, and delayed phases, and it is often carried out after detection of an abnormality on ultrasound. The typical CT findings of HCC during the arterial phase 25 seconds after contrast injection are increased enhancement of the tumor as compared with nontumorous liver parenchyma. Seventy seconds after contrast injection, during the portal venous phase, the lesion is either isodense or hypodense; 300 seconds later, during the delayed phase, HCC is typically hypodense due to the early “washout” of contrast.18
MRI has been shown to be more accurate than CT in the detection of neoplastic lesions. Gadolinium-enhanced MRI demonstrates a hyperintense image of the tumor in the arterial phase, isointensity in the portal phase, and hypointensity in the delayed phase. T2-weighted images typically demonstrate hyperintensity; T1-weighted images reveal variable intensity. Sensitivity of MRI and CT in detecting HCC has been noted to be 81% and 68%, respectively, and specificity of MRI and CT is 85% and 93%, respectively. MRI can more reliably differentiate HCC from regenerating or dysplastic nodules as compared with CT.19,20
Biopsy
Percutaneous biopsy confirmation of HCC should only be performed if radiologic studies demonstrate uncertainty in the diagnosis, as there are several risks associated with biopsy such as needle track seeding and bleeding.21 Needle core biopsy is preferred over fine needle aspiration biopsy (FNAB), since it provides a more reliable specimen.22 FNAB has been reported to have high false-negative rates and is highly dependent on the expertise of the cytopathologist.23 However, FNAB has the advantages of being a less invasive test lowering the complication rates of this procedure, as well as providing for an instant assessment of whether sufficient tissue samples were obtained.24
The risk of malignant needle track seeding is a recognized complication of biopsy, and is clinically significant in patients who are under consideration for liver transplantation (LT) or resection. A recent review reported 14 series with 66 cases of seeding following biopsy. The risk of seeding ranged from 0% to 11%, with a median value of 2.29%.25 Numerous factors have been related to the risk of neoplastic dissemination: larger diameter needles, more passes, superficial location of the tumor in the liver, intrinsic metastatic property of the tumor related to either or both tumor size/aggressiveness, and patients’ immunodepression resulting in reduced tumor surveillance.
Serology
Although the use of alpha-fetoprotein (AFP) has been used for both surveillance and diagnosis in the past, recent studies have discounted its utility. The Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) trial analyzed the accuracy of both AFP and descarboxyprothrombin (DCP) in diagnosing HCC among 1031 randomized patients with hepatitis C-related cirrhosis. Among this group, 39 patients developed HCC over the course of the study; however, neither biomarker alone was sufficiently sensitive or specific for the detection of HCC, warranting the continued need for radiological or histological diagnosis.26 Serum AFP levels have been shown to be normal in patients with fibrolamellar HCC, which generally occurs in young adults without underlying cirrhosis.27 Furthermore, elevations in serum AFP are noted in a number of other diseases such as intrahepatic cholangiocarcinoma or other nonmalignant conditions, and the serum level rarely correlates with size, stage, or prognosis.
Surveillance
HCC is unique among cancers in that it is usually preceded by chronic liver disease and cirrhosis, thus providing a well-defined target population for whom surveillance strategies may be devised. Unfortunately, many questions remain about the feasibility and efficacy of surveillance for HCC. In order to make informed decisions about which patients should be entered into a surveillance program for HCC, we need to address some important issues:
The ideal objective would be to decrease mortality from the disease. There is a single randomized controlled study of surveillance versus no surveillance that has shown a survival benefit. This study that was conducted in China and recruited 18,816 at-risk patients demonstrated a 37% reduction in HCC-related mortality. Due to adherence rates of only 60%, this result probably represents the minimum benefit that may be expected from surveillance, and ideally should be replicated in other study populations. However, ethical issues probably preclude such a study being performed in the future.28
The decision to enter a patient into a surveillance program is determined by the level of risk for HCC. There is no population-based data estimating such a risk, and therefore current guidelines are based on decision analysis studies. In general, surveillance for HCC in cirrhosis of various etiologies was found to be cost-effective if the risk of HCC was 1.5%/year or greater. The only exceptions to this are patients with chronic HBV infection who develop HCC even in the absence of cirrhosis. Analysis of this population suggests that surveillance was cost-effective when the incidence of HCC exceeds 0.2%/year,11 see Table 7.1.
An additional group of patients who should undergo surveillance for HCC are patients on the liver transplant waiting list, as current United Network of Organ Sharing (UNOS) criteria gives priority for transplantation to patients who develop HCC. Conversely, identification of a neoplastic lesion that exceeds guidelines during the waiting period would result in the elimination of that patient from transplant candidacy.11
Screening or surveillance tests for HCC fall into two categories—serological and radiological. Of the serological tests, the performance characteristics of AFP have been best studied. Despite its widespread use for HCC surveillance, AFP has suboptimal accuracy, with sensitivity of 66% and specificity of 82%. As such, its continued utility as a tumor marker for HCC has been questioned. Other serological tests are DCP, glypican 3, and heat-shock protein 70, as well as the ratio of glycosylated AFP (L3 fraction) to total AFP. None of these can be recommended as a screening test at the current time.
Table 7.1 Recommended surveillance populations and the associated annual incidence of hepatocellular carcinoma.
| Population group for which surveillance is recommended | HCC incidence/year |
HBV carrier
| 0.4–0.6% 0.3–0.6% Unclear but higher than those without family history Occurs at younger age |
Cirrhosis due to
| 3–8% 3–5% 3–5% Unknown but >1.5%/year Unknown but >1.5%/year |
| Source: Reproduced with permission from the American Association for the Study of Liver Diseases. Bruix, J., Sherman, M. (2010) Management of Hepatocellular Carcinoma: An Update. American Association for the Study of Liver Diseases, Alexandria, VA. This updates a previous version: Bruix J, Sherman M. Management of Hepatocellular Carcinoma. Hepatology 2005 42(5), 1208–1236. | |
The radiological test most commonly used for surveillance is ultrasonography. Ultrasound is reported to have a sensitivity of 65–80% and specificity of more than 90% when used for screening. Its performance characteristics are negatively impacted by the presence of cirrhotic nodules, subject obesity, and operator inexperience. However, its easy availability and relative cost-efficacy make it the test of choice for surveillance. Surveillance intervals should be between 6 and 12 months based on reported tumor doubling times.
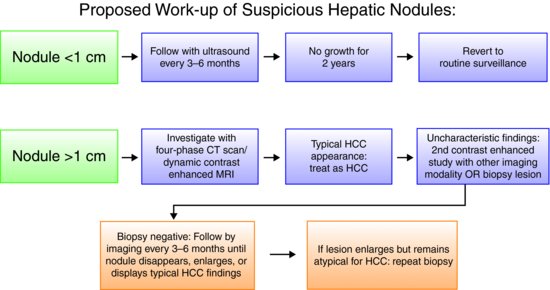
The latest update to the AASLD practice guidelines for management of patients with HCC based on surveillance screening is summarized in Figure 7.1.
Figure 7.1 Biopsies of small lesions should be evaluated by expert pathologists. Tissue that is not clearly HCC should be stained with all the available markers including CD34, CK7, glypican 3, HCP-70, and glutamine synthetase to improve diagnostic accuracy. (Adapted from AASLD Practice Guidelines.11)
Prognosis and staging
Cancer staging systems are vital prognostic tools, and guide treatment decisions while stratifying different key factors into a common algorithm. Many different staging systems exist for HCC and there is no uniform consensus for the use of any single system. Most of these staging systems take into account the following parameters: aggressiveness and growth rate of the tumor, the presence of vascular invasion, hepatic synthetic function, and performance status. The most commonly used staging systems are the Barcelona Clinic Liver Cancer (BCLC) staging classification, the American Joint Committee on Cancer Tumor Node Metastasis (AJCC-TNM) system, the Okuda system, and the Cancer of the Liver Italian Program (CLIP) system.29–32 AASLD Practice Guidelines recommend the use of the BCLC staging system for patients with HCC (Figure 7.2).11 The BCLC system is unique in that it incorporates tumor stage, liver function as defined by Child–Turcotte–Pugh (CTP) class, and performance status, linking these to individualized treatment modalities. This staging system’s ability to stratify patients into different prognostic categories has been well documented in a meta-analysis of untreated patients with HCC in randomized clinical trials, as well as other major trials of HCC therapy.33–36









