(1)
Northeast Ohio Medical University, Rootstown, OH, USA
(2)
Department of Anesthesiology, Stony Brook University, Stony Brook, NY, USA
Keywords
Postoperative nausea and vomitingAntiemeticsTotal intravenous anesthesia5-HT3 receptor antagonistsCorticosteroidsNeurokinin-1 receptor antagonistsAnticholinergicsProphylactic treatment with antiemeticsPostoperative nausea and vomiting (PONV) are common and unpleasant complications of anesthesia and surgery. The overall incidence rate of PONV for all surgical patients is estimated to be 25–30 %, while the rate of PONV in high-risk patients can be as high as 80 % [1–4]. An estimated 0.18 % of patients experience intractable PONV, which may result in prolonged postanesthesia care unit (PACU) stay, unanticipated hospital readmission, and increased health care costs [5–7]. PONV represents one of the most common reasons for poor patient satisfaction scores in the postoperative period [8]. One survey found that patients would be willing to pay up to $100, at their own expense, for complete and effective antiemetic treatment [9].
The aim of this chapter will be to summarize the evidence for the implementation of PONV protocols within an enhanced recovery after surgery program (ERP). Literature used for these recommendations come from randomized control trials, meta-analyses, and consensus guidelines. We will address the following: identifying high-risk patients, minimizing risks, administering appropriate prophylactic antiemetic and rescue treatment, and recommending a treatment algorithm for use in an ERP protocol.
Identifying High-Risk Patients
There are several factors that have been associated with increased risk of PONV, but to effectively stratify risk, one should focus on those factors that independently predict PONV. These factors include female sex, history of PONV or motion sickness, nonsmoking status, younger age, general versus regional anesthesia, use of volatile anesthetics and nitrous oxide, postoperative opioids, duration of surgery, and type of surgery (cholecystectomy, laparoscopic, gynecological) [10]. The increased incidence of PONV with laparoscopic surgeries and cholecystectomies is particularly relevant when considering ERP protocols for gastrointestinal surgery [11]. To ease the task in risk stratification, Apfel et al. [1] developed a simplified risk score, based on four predictors: female gender, history of motion sickness or PONV, nonsmoking status, and the use of opioids for postoperative analgesia. The incidence of PONV in patients with 0, 1, 2, 3, or 4 of these risk factors was 10, 21, 39, 61, and 79 % respectively (Fig. 8.1) [1]. The use of this simplified risk score to guide therapeutic interventions has been shown to dramatically reduce institutional rates of PONV [12–14] (Fig. 8.2).
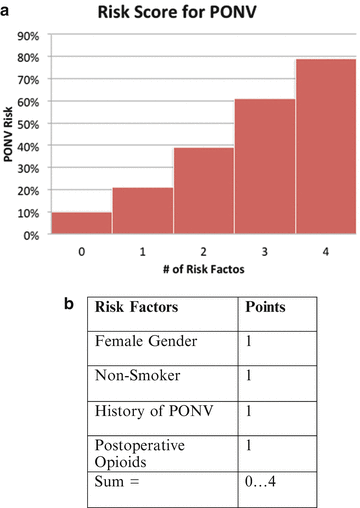
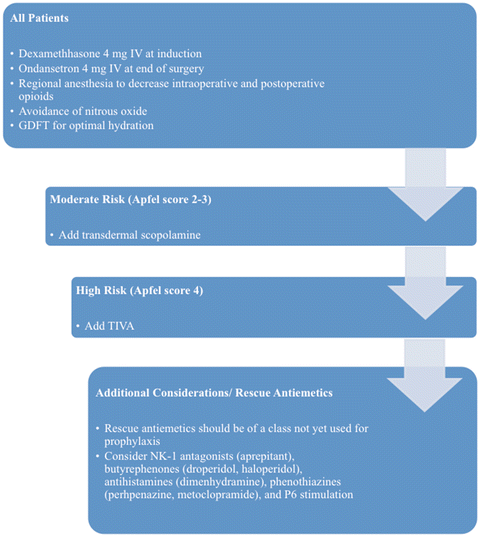

Fig. 8.1.
(a) Risk score for PONV. (b) Risk factors contribute to simplified risk score from Apfel et al. These can be used to predict a patient’s risk for PONV. (A, From Apfel, C.C., et al., A Simplified Risk Score for Predicting Postoperative Nausea and Vomiting: Conclusions from Cross‐validations between Two Centers. Anesthesiology, 1999. 91(3): p. 693, with permission).

Fig. 8.2.
Treatment algorithm for preventing PONV for patients in an ERP protocol.
Reducing Baseline Risks
There are several strategies that can be used to reduce the baseline risk of PONV: Avoiding general anesthesia by the use of regional anesthesia; Using propofol, an antiemetic in its own right, for induction and maintenance of anesthesia; Avoiding nitrous oxide; Avoiding volatile anesthetics; Minimizing intraoperative and postoperative opioids; and adequate hydration [10]. The complete avoidance of general anesthesia is not generally practical for gastrointestinal surgery; however, the use of transversus abdominis plane (TAP) blocks as part of the analgesic regimen reduced incidence of PONV in colonic surgery patients [15]. This same effect has not been shown for epidural analgesia [16, 17]. This could be related to the amount of opioid used. Similarly, propofol for induction and maintenance of anesthesia (total IV anesthesia [TIVA]) has been shown to reduce the risk of PONV by approximately 25 % [14]. Additionally, two meta-analyses have shown that omitting nitrous oxide reduced both early and late PONV, except when baseline risks were already low [18, 19]. Early PONV, specifically, may be reduced by avoiding volatile anesthetics, as they have been identified as the primary cause of early PONV [20].
Another primary cause of PONV can be avoided by reducing or minimizing postoperative opioids [1, 19–24]. To achieve adequate analgesia without opioids, one can utilize several modalities including regional or neuraxial analgesia, opioid adjuncts such as nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase-2 (COX-2) inhibitors, acetaminophen, calcium antagonists (gabapentin, pregabalin), and NMDA receptor antagonists (ketamine). NSAIDs and COX-2 inhibitors have been shown, in randomized controlled trials and meta-analyses, to have a morphine-sparing effect in the postoperative period [25–27]. To a lesser degree, ketamine may offer a similar morphine-sparing effect [28].
The volume and type of fluid administered in the perioperative period can influence the incidence of PONV and bowel function. Optimal fluid management in an ERP protocol involves judicious administration of background crystalloids, while optimizing hemodynamics with colloids as part of a goal-directed fluid therapy (GDFT) [29]. A meta-analysis shows that GDFT, aimed at maximizing flow-related hemodynamic values, reduces PONV, as well as hospital stay, complications, and ileus [30]. Meanwhile, Gustafsson et al. [31] showed that fluid overload can increase complications, particularly cardiovascular complications, in colorectal patients. This same study also showed that preoperative carbohydrate drinks, as part of an ERP protocol, can significantly reduce PONV [31]. It is therefore recommended that to minimize PONV in an ERP protocol, excessive crystalloids administration should be avoided. GDFT strategy based on hemodynamic variables should be available in patients undergoing major or high-risk surgery.
PONV Prophylaxis
Prophylactic treatment with antiemetics is a key aspect of PONV prevention. Several classes of antiemetics exist for this purpose and each will be discussed in turn; the benefits and side effects of these classes are listed in Table 8.1. Recommended doses and times of administration for specific antiemetics are listed in Table 8.2.
Table 8.1.
Benefits and side effects of the main classes of PONV prophylactic antiemetics and alternative treatment strategy.
Antiemetic class | Benefits | Side effects |
|---|---|---|
5-HT3 receptor antagonists (e.g., ondansetron, dolasetron, granisetron, tropisetron) | Specific for PONV Do not have sedative side effects | Headache, constipation, elevated liver enzymes, risk of QT prolongation |
NK-1 receptor antagonists (e.g., aprepitant, casopitant, rolapitant) | Long duration of action Improved efficacy against vomiting Do not have sedative side effects | Headache, constipation |
Corticosteroids (e.g., dexamethasone, methylprednisolone) | Do not have sedative side effects Long duration of action | Hyperglycemia in obese and diabetic patients, may increase risk of wound infection |
Butyrophenones (e.g., droperidol, haloperidol) | Improved prophylaxis against nausea | Sedation with high doses, hypotension, extrapyramidal side effects, neuroleptic malignant syndrome, droperidol has an FDA “black box” warning regarding QTc prolongation. However the risk is considered minimal with antiemetic doses |
Antihistamines (e.g., dimenhydrinate, meclizine) | Effective against motion sickness Meclizine has longer duration of action | Sedation, dry mouth, restlessness |
Anticholinergics (e.g., scopolamine) | Effective against motion sickness Transdermal preparation has long duration of action and can be applied the night before surgery | Sedation, visual disturbances, dry mouth, dizziness, restlessness, central cholinergic syndrome |
Phenothiazines (e.g., perphenazine) | Long duration of action | Extrapyramidal side effects, hypotension, restlessness, anticholinergic syndrome, may cause sedation |
Acupuncture (P6 stimulation) | Improved efficacy against nausea | None reported when used for PONV prophylaxis |
TIVA with Propofol | Effective against early PONV Reduces the incidences of PDNV | Increased risk of awareness |
Table 8.2.
Antiemetic doses and timing for prevention of PONV.
Drug | Dose | Timing |
|---|---|---|
Aprepitant | 40 mg per os | At induction |
Casopitant | 150 mg per os | At induction |
Dexamethasone | 4–5 mg IV | At induction |
Dimenhydrinate | 1 mg/kg IV | |
Droperidola | 0.625–1.25 mg IV | End of surgery |
Granisetron | 0.35–3 mg IV | End of surgery |
Haloperidol | 0.5–<2 mg IM/IV | |
Methylprednisolone | 40 mg IV | |
Ondansetron | 4 mg IV, 8 mg ODT | End of surgery |
Palonosetron | 0.075 mg IV | At induction |
Perphenazine | 5 mg IV | |
Ramosetron | 0.3 mg IV | End of surgery |
Rolapitant | 70–200 mg per os | At induction |
Scopolamine | Transdermal patch | Prior evening or 2 h before surgery |
Tropisetron | 2 mg IV | End of surgery |
5-HT3 Receptor Antagonists
The 5-HT3 antagonists are a unique group of drugs that were developed specifically for the management of nausea and vomiting. In general, their antiemetic (anti-vomiting) effects are stronger than their anti-nausea effects [32]. Most of the available research on this class of drugs focuses on ondansetron. A dose of 4 mg ondansetron has a number needed to treat (NNT) of approximately 6 for prevention of vomiting (0–24 h) and approximately 7 for prevention of nausea [32]. The 8 mg oral disintegrating tablet (ODT) is equally effective to the 4 mg IV dose [33]. Other 5-HT3 antagonists which have been shown to be effective in preventing PONV include: granisetron 0.35–3 mg IV [34], tropisetron 2 mg IV [35], ramosetron 0.3 mg IV [36, 37], and palonosetron 0.075 mg IV [38, 39]. Of these, tropisetron and ramosetron are not available in the United States.
One reason 5-HT3 antagonists are widely used for the prevention of PONV is their favorable side effect profile. All, except palonosetron, have been shown to prolong the QTc interval [10]. For ondansetron specifically, the U.S. FDA recommends that the dose should not exceed 16 mg in a single dose because of the risks to the QTc interval. The number-needed-to-harm (NNH) for a single dose of ondansetron is 36 for headache, 31 for elevated liver enzymes, and 23 for constipation [19]. All 5-HT3 antagonists are considered to be equally safe.
Corticosteroids
Dexamethasone 4–5 mg IV has widely been used prophylactically to prevent PONV. It has been shown to be especially effective against late PONV [40]. A recent meta-analysis showed that the NNT for TIVA over 24 h was 3.7 (95 % CI, 3.0–4.7) [41]. There is also the added benefit that dexamethasone 0.1 mg/kg has been shown to be an effective adjunct in multimodal strategies to reduce postoperative pain and opioid consumption [42]. Recently, an increasing number of studies have used a higher dose of dexamethasone 8 mg IV. One such study found that preoperative dexamethasone 8 mg enhances the postdischarge quality of recovery in addition to reducing nausea, pain, and fatigue [43]. However, a recent meta-analysis showed no clinical advantage of higher dose 8–10 mg IV dexamethasone compared with 4–5 mg IV [41].
There is conflicting data concerning the safety of dexamethasone. Most studies show that a single dose of perioperative dexamethasone does not appear to increase the risk of wound infection [40, 42]. However, dexamethasone has been shown to cause significant increases in blood glucose levels that occur 6–12 h postoperatively in normal subjects [44, 45], those with impaired glucose tolerance [45], type 2 diabetics [46], and obese patients [45]. Additionally, one recent retrospective case-control study showed that patients who developed a postoperative wound infection were significantly more likely to have received a single perioperative dose of dexamethasone 4–8 mg IV, and thus concluded that dexamethasone may increase the postoperative risk of wound infection [47]. However, these patients were also less likely (p = 0.001) to have received a prophylactic antibiotics. Based on the totality of evidence, a single dose of 4–8 mg dexamethasone is not associated with an increased risk and hence is recommended [48, 49]. The increase in blood sugar is predictable, and should be monitored in labile diabetics. Methylprednisolone 40 mg IV is also effective for the prevention of late PONV, as it has a similarly long half-life [50, 51]. There is no evidence to suggest that methylprednisolone differs from dexamethasone in terms of adverse effects.
Butyrophenones
Droperidol and haloperidol are two butyrophenones that have been shown to be effective for the prophylactic prevention of PONV. An effective dose of droperidol for preventing PONV is 0.625–1.25 mg IV [52–54]. It has been shown to have similar efficacy to ondansetron, with an NNT of approximately 5 for the prevention of PONV within 24 h [54]. However, in 2001, the FDA issued a black box restriction on the use of droperidol over concerns of QTc prolongation. Despite this, studies have shown that droperidol has equal effects on the QTc interval as ondansetron [55, 56]. Furthermore, the combination of droperidol and ondansetron, while being more effective than either drug alone for the prevention of PONV, had no greater effect on the QTc interval than either drug alone [57]. It is believed, therefore, that at dosing levels appropriate for the prevention of PONV, droperidol can be safely used without significant cardiovascular events.
At low doses, 0.5–2 mg IM or IV, haloperidol effectively reduced the risk of PONV with an NNT between 4 and 6 [58]. Haloperidol carries a warning of QTc prolongation on its label; however at these low doses cardiac arrhythmias have not been reported. Nonetheless, haloperidol is not regarded as a first antiemetic of choice, as it also carries risk of extrapyramidal symptoms. The use of haloperidol for PONV and the IV route of administration is not an FDA-approved indication.
Antihistamines
Antihistamines are older drugs with antiemetic effects. The antihistamine dimenhydrinate can be used as an antiemetic in doses of 1 mg/kg IV, where it has shown to have similar efficacy to the 5-HT3 receptor antagonists, dexamethasone, and droperidol [59, 60]. Despite this, there are too few direct comparisons with other antiemetics; furthermore, there is not enough data to determine optimal administration timing, dose response, or side effect profile [10].
Anticholinergics
Transdermal scopolamine (TDS) has the added benefit that it can be applied the evening before surgery or 2–4 h before the start of anesthesia because of its 2- to 4-h onset of effect [61, 62]. TDS has been shown to have equal effectiveness in single drug therapy studies comparing it to ondansetron and droperidol [63]. TDS prevented nausea and vomiting up to 24 h after surgery with an NNT of 6 [64]. The most common adverse effects include visual disturbances (NNH = 5.6), dry mouth (NNH = 13), and dizziness (NNH = 50) [64].
Phenothiazines
Perphenazine and metoclopramide are two phenothiazines that have been used for the management of PONV. A review of 6 RCTs showed a relative risk reduction (RRR) of 0.5 (95 % CI, 0.37–0.67) for PONV when using 5 mg IV perphenazine [65]. This same review showed no significant increase in sedation or drowsiness when compared with a placebo [65]. Metoclopramide is not effective at 10 mg dose. However, efficacy has been demonstrated at higher doses. The NNT for metoclopramide 10, 25, and 50 mg for PONV at 24 h is 30, 16, and 11, respectively [66]. The NNH for extrapyramidal symptoms with 25 or 50 mg metoclopramide is 140 [66].
NK-1 Receptor Antagonists
The most widely studied of the neurokinin-1 (NK-1) receptor antagonists and the only one currently available is aprepitant. Compared with ondansetron, aprepitant has a longer duration of action, with a half-life of 9–13 h [67]. While it was shown to be similar to ondansetron in achieving complete response (no vomiting and no use of rescue antiemetic) for 24 h after surgery, it was shown to be more effective than ondansetron at preventing vomiting at 24 and 48 h after surgery and in reducing nausea severity during the first 48 h after surgery [68, 69]. The side effect profile of aprepitant is similar to that of ondansetron [69]. The role of aprepitant as a routine prophylactic agent has not yet been established due to limited clinical experience and higher costs [70]. Casopitant and rolapitant are similar long-acting NK-1 antagonists which have shown comparable efficacy, but have not yet been approved for use [10].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





