Antenatal sonography has markedly increased the detection of urogenital anomalies, including those conditions that lead to significant morbidity and mortality. Prenatal intervention is feasible to arrest and sometimes reverse the sequelae of bladder outlet obstruction but not necessarily renal damage. Myelomeningoceles, the most severe form of spina bifida, can be corrected in utero, with improvements in hydrocephalus seen along with a decreased incidence of ventricular shunting postnatally. Medical therapy to prevent virilization associated with congenital adrenal hyperplasia has been successful, with improved ability to detect its presence prenatally now possible. As further techniques evolve to correct underlying disease processes, it becomes important to critically assess the therapies, particularly with long-term outcome data.
With the advent of maternal-fetal screening ultrasonography in the mid-1980s, the ability to identify a variety of congenital anomalies in utero has provided not only an early glimpse into the developmental window of several devastating congenital conditions but also the tantalizing opportunity to intervene at a time that may dramatically alter the long-term prognosis and outcomes. It is estimated that a structural fetal anomaly is detected by antenatal sonography in 1% of all screened pregnancies. Of these anomalies, a fifth are believed to be manifestations of genitourinary origin, second only to those found within the central nervous system. Depending on the sonographic criteria used, anomalies of the genitourinary tract are believed to occur as frequently as 1 out of every 100 pregnancies, with hydronephrosis being the most common finding detected.
As prenatal management has evolved, many questions have emerged regarding not only the indications for therapeutic interventions, but more importantly the relative merits of fetal intervention. In this review, the authors provide a historical background of the development of fetal therapeutic interventions and an overview of current fetal interventions for antenatally diagnosed genitourinary tract disorders.
The development of fetal intervention
Significant advances in the past quarter century have moved the concept of therapeutic fetal intervention into the realm of not just what is possible, but even what may be considered by some, routine. To meet the evolving needs of families facing the dilemma of a fetus diagnosed with a significant structural anomaly, several institutions have established specialized centers dedicated to the comprehensive diagnosis and treatment of fetal anomalies. Today’s multidisciplinary team approach to fetal care often includes dedicated providers representing maternal-fetal medicine, pediatric anesthesia, pediatric surgery, pediatric radiology, pediatric urology, and pediatric nursing.
The earliest examples of modern fetal therapeutic intervention included the use of transfusions for erythroblastosis fetalis, the pharmacologic management of fetal cardiac arrhythmias, and the percutaneous drainage of fetal hydrothorax. The correction of more complex anatomic defects such as congenital diaphragmatic hernias followed soon after, necessitating the move to the more invasive techniques of direct exposure via hysterotomy and open fetal surgical repair.
Early pioneers of open fetal surgery faced several technical and ethical challenges regarding the maternal and fetal risks posed by open fetal surgery using midgestational hysterotomy. The group from the University of California San Francisco (UCSF) proposed the following prerequisites for undertaking open fetal surgery: (1) the natural history of the disease in question must be thoroughly established by careful examination of untreated cases; (2) a strict selection criteria must be developed identifying appropriate candidates for fetal intervention; (3) the pathophysiology and potential benefits of fetal intervention must first be demonstrated in appropriate animal models; and (4) hysterotomy and open fetal surgery must be accomplished without undue risk to the mother and her future reproductive potential.
The early results of fetal therapy from UCSF included the initial experience with 17 patients who had 1 of 4 indications: (1) bilateral hydronephrosis with presumed lower urinary tract obstruction (LUTO, n = 7); (2) congenital diaphragmatic hernia (CDH, n = 8); (3) sacrococcygeal teratoma (SCT, n = 1); and (4) congenital cystic adenomatoid malformation (CCAM, n = 1). Of the 7 patients with suspected LUTO, 2 died at shortly after birth as a result of complications related to pulmonary hypoplasia, 1 died of septicemia with normal renal function within the first year of life, and 2 were alive at follow-up, although 1 child had manifested renal deterioration. One fetus was removed because of inadequate repair of a coexisting cloacal anomaly and 1 was delivered 12 days following repair when the mother opted to discontinue tocolytics. These early mixed results reinforced the notion that anomalies that may be amenable to correction by hysterotomy and open fetal repair should only be repaired in the context of an acceptable level of risk posed to the mother’s health and future reproductive potential. To this end, concerns about maternal risk focused on 3 main areas: (1) the operative risk of general anesthesia and a midgestational hysterotomy; (2) the risk of premature labor following hysterotomy; and (3) the risk of compromising future maternal reproductive potential. The biggest challenge of the early era of fetal surgery was the ability to manage uterine contractions and premature labor following hysterotomy. Despite tocolytic therapy, all mothers developed preterm labor ranging from 25 to 35 week’s gestation. In addition, those women who underwent successful hysterotomy and fetal intervention were subsequently committed to delivering by cesarean section, essentially undergoing 2 hysterotomies during the course of a single pregnancy. The fetal therapy team at UCSF cautioned that simply because these procedures were technically feasible did not mean that they should be performed. They further added that it is critical to vigorously examine the efficacy, safety, and cost-effectiveness of fetal therapy compared with conventional conservative treatment. Even as the overall techniques of fetal surgery have improved, these early sentiments are still true today in evaluating the appropriateness of fetal intervention.
Antenatal hydronephrosis/LUTO
Of the approximately 1% of screened pregnancies that are found to have a genitourinary tract anomaly, more than 50% represent a diagnosis of antenatal hydronephrosis, making it 1 of the most common fetal anomalies detected. Although antenatal hydronephrosis frequently amounts to little in the way of clinical significance in most cases, it is important to identify the small subset of fetuses that present with a significant LUTO and are at risk for significant postnatal morbidity and mortality.
The identification of antenatal hydronephrosis is made on screening ultrasonography by the demonstration of renal pelvic and calyceal dilatation. Antenatal hydronephrosis does not reflect a singular diagnosis, but rather the clinical manifestation of a heterogeneous group of pathologic and physiologic entities that lead to the appearance of urinary tract dilatation. Potential causes include physiologic dilatation, vesicoureteral reflux, anomalies of the ureteropelvic or ureterovesical junction, primary obstructed megaureters, multicystic dysplastic kidneys, ureteral ectopia, posterior urethral valves, prune-belly syndrome, urethral atresia, and cloacal anomalies. The following list provides a differential diagnosis for the fetus with antenatal hydronephrosis
Anomalous ureteropelvic junction/ureterovesical junction obstruction
Multicystic kidney
Primary obstructive megaureter
Vesicoureteral reflux (bladder may be distended)
Ectopic ureterocele (bladder may be distended)
Ectopic ureter
Physiologic dilatation
Posterior urethral valves (bladder may be distended)
Prune-belly syndrome (bladder may be distended)
Urethral atresia (bladder may be distended)
Hydrocolpos (bladder may be distended)
Pelvic tumor (bladder may be distended)
Cloacal anomaly (bladder may be distended).
Technical issues that may affect the identification of hydronephrosis antenatally include the operator-dependent nature of ultrasonography and the timing of the study with respect to gestational age. Typically, the ability to detect urinary tract dilatation is better later in gestational age because the fetus is not only larger but significant obstructive anomalies are also more readily appreciated with progressive dilatation. The severity of hydronephrosis and the point at which it occurs in gestation may help indicate the likelihood for urinary obstruction and subsequent need for surgical intervention.
Once hydronephrosis has been recognized, management consists of distinguishing clinically significant urinary tract obstruction that poses an imminent risk to the fetus from conditions that may be addressed in the postnatal period. In helping to make this assessment, consideration should be given to the presence of bilateral hydronephrosis, coexisting ureteral and bladder distention, hydronephrosis onset, and the timing and severity of oligohydramnios.
The presence of unilateral hydronephrosis typically requires no specific interventions during the prenatal period beyond close serial imaging. Bilateral hydronephrosis, on the other hand, can be present in the context of clinically significant urinary tract obstruction such as posterior urethral valves or urethral atresia, as well as in nonobstructing entities such as prune-belly syndrome or high-grade vesicoureteral reflux. The development of in utero ureteropelvic junction obstruction may compromise renal function depending on timing and severity, but the neonatal kidney often retains a tremendous capacity for improvement following postnatal pyeloplasty. Even in the case of bilateral in utero ureteropelvic junction obstruction, rarely will amniotic fluid volumes be compromised to a degree that would raise concern for the development of pulmonary hypoplasia. The same is true for primary obstructed megaureters. Thus, fetal interventions such as percutaneous drainage of the fetal kidney or early delivery for immediate urologic surgery in these cases are not warranted.
The ominous constellation of bladder distention, impaired bladder emptying, bilateral hydroureteronephrosis, and oligohydramnios represents a significant LUTO that often results in fetal morbidity and mortality. LUTO is predominately associated with male fetuses that are most commonly diagnosed with either posterior urethral valves or urethral atresia. Other causes include prune-belly syndrome, anterior urethral valves, congenital urethral hypoplasia, prolapsing ureteroceles, and cloacal plate anomalies in females. Variable degrees of renal impairment, reduction in amniotic fluid, and subsequent development of pulmonary hypoplasia can be seen depending on the severity and duration of urinary tract obstruction. Progressive renal deterioration manifests as the appearance of fibrous echogenic kidneys and cystic degeneration of fetal kidneys. Animal models support the hypothesis that renal fibrocystic dysplasia occurs in the presence of midgestational urinary tract obstruction as opposed to later in gestation. In humans, the onset of midgestational (second trimester) oligohydramnios is suspected to lead to the development of renal dysplasia. The resulting reduction in production of amniotic fluid leads to the development of either oligohydramnios, or in the extreme setting, anhydramnios. Reduced amniotic fluid volumes are associated with the development of pulmonary hypoplasia, which represents the leading cause of perinatal mortality in cases of LUTO. Complete obstruction, as seen in urethral atresia, is nearly always fatal because of pulmonary hypoplasia. Prune-belly syndrome, although generally considered nonobstructive in nature, can also lead to the development of renal insufficiency postnatally.
Antenatal hydronephrosis/LUTO
Of the approximately 1% of screened pregnancies that are found to have a genitourinary tract anomaly, more than 50% represent a diagnosis of antenatal hydronephrosis, making it 1 of the most common fetal anomalies detected. Although antenatal hydronephrosis frequently amounts to little in the way of clinical significance in most cases, it is important to identify the small subset of fetuses that present with a significant LUTO and are at risk for significant postnatal morbidity and mortality.
The identification of antenatal hydronephrosis is made on screening ultrasonography by the demonstration of renal pelvic and calyceal dilatation. Antenatal hydronephrosis does not reflect a singular diagnosis, but rather the clinical manifestation of a heterogeneous group of pathologic and physiologic entities that lead to the appearance of urinary tract dilatation. Potential causes include physiologic dilatation, vesicoureteral reflux, anomalies of the ureteropelvic or ureterovesical junction, primary obstructed megaureters, multicystic dysplastic kidneys, ureteral ectopia, posterior urethral valves, prune-belly syndrome, urethral atresia, and cloacal anomalies. The following list provides a differential diagnosis for the fetus with antenatal hydronephrosis
Anomalous ureteropelvic junction/ureterovesical junction obstruction
Multicystic kidney
Primary obstructive megaureter
Vesicoureteral reflux (bladder may be distended)
Ectopic ureterocele (bladder may be distended)
Ectopic ureter
Physiologic dilatation
Posterior urethral valves (bladder may be distended)
Prune-belly syndrome (bladder may be distended)
Urethral atresia (bladder may be distended)
Hydrocolpos (bladder may be distended)
Pelvic tumor (bladder may be distended)
Cloacal anomaly (bladder may be distended).
Technical issues that may affect the identification of hydronephrosis antenatally include the operator-dependent nature of ultrasonography and the timing of the study with respect to gestational age. Typically, the ability to detect urinary tract dilatation is better later in gestational age because the fetus is not only larger but significant obstructive anomalies are also more readily appreciated with progressive dilatation. The severity of hydronephrosis and the point at which it occurs in gestation may help indicate the likelihood for urinary obstruction and subsequent need for surgical intervention.
Once hydronephrosis has been recognized, management consists of distinguishing clinically significant urinary tract obstruction that poses an imminent risk to the fetus from conditions that may be addressed in the postnatal period. In helping to make this assessment, consideration should be given to the presence of bilateral hydronephrosis, coexisting ureteral and bladder distention, hydronephrosis onset, and the timing and severity of oligohydramnios.
The presence of unilateral hydronephrosis typically requires no specific interventions during the prenatal period beyond close serial imaging. Bilateral hydronephrosis, on the other hand, can be present in the context of clinically significant urinary tract obstruction such as posterior urethral valves or urethral atresia, as well as in nonobstructing entities such as prune-belly syndrome or high-grade vesicoureteral reflux. The development of in utero ureteropelvic junction obstruction may compromise renal function depending on timing and severity, but the neonatal kidney often retains a tremendous capacity for improvement following postnatal pyeloplasty. Even in the case of bilateral in utero ureteropelvic junction obstruction, rarely will amniotic fluid volumes be compromised to a degree that would raise concern for the development of pulmonary hypoplasia. The same is true for primary obstructed megaureters. Thus, fetal interventions such as percutaneous drainage of the fetal kidney or early delivery for immediate urologic surgery in these cases are not warranted.
The ominous constellation of bladder distention, impaired bladder emptying, bilateral hydroureteronephrosis, and oligohydramnios represents a significant LUTO that often results in fetal morbidity and mortality. LUTO is predominately associated with male fetuses that are most commonly diagnosed with either posterior urethral valves or urethral atresia. Other causes include prune-belly syndrome, anterior urethral valves, congenital urethral hypoplasia, prolapsing ureteroceles, and cloacal plate anomalies in females. Variable degrees of renal impairment, reduction in amniotic fluid, and subsequent development of pulmonary hypoplasia can be seen depending on the severity and duration of urinary tract obstruction. Progressive renal deterioration manifests as the appearance of fibrous echogenic kidneys and cystic degeneration of fetal kidneys. Animal models support the hypothesis that renal fibrocystic dysplasia occurs in the presence of midgestational urinary tract obstruction as opposed to later in gestation. In humans, the onset of midgestational (second trimester) oligohydramnios is suspected to lead to the development of renal dysplasia. The resulting reduction in production of amniotic fluid leads to the development of either oligohydramnios, or in the extreme setting, anhydramnios. Reduced amniotic fluid volumes are associated with the development of pulmonary hypoplasia, which represents the leading cause of perinatal mortality in cases of LUTO. Complete obstruction, as seen in urethral atresia, is nearly always fatal because of pulmonary hypoplasia. Prune-belly syndrome, although generally considered nonobstructive in nature, can also lead to the development of renal insufficiency postnatally.
Indications for prenatal therapy
Over the years, criteria have been established to identify which fetuses with LUTO would benefit from prenatal intervention. The goals were to exclude patients who were unlikely to benefit from therapeutic intervention based on the coexistence of other significant chromosomal or structural anomalies and/or advanced renal fibrocystic dysplasia that would preclude measurable improvement. The selection of candidates for fetal intervention typically includes (1) a fetal karyotype to exclude chromosomal abnormalities, (2) an ultrasound to detect other structural anomalies that may adversely affect prognosis, and (3) serial fetal urine analyses to assess the extent of renal impairment. Reported unfavorable prognostic risk factors include (1) prolonged oligohydramnios, (2) presence of renal cortical cysts, (3) urinary sodium level greater than or equal to 100 mEq/L, chloride level more than 90 mEq/L, osmolarity more than 210 mmol/L and increased urinary β2-microglobulin level (>6 mg/L), and (4) reduced lung area and/or thoracoabdominal circumference.
More recently, 2 systematic reviews have been conducted evaluating the existing literature on the accuracy of fetal urine analysis and antenatal ultrasonography in predicting poor postnatal renal function. Both reviews are limited by the poor methodological quality of studies available. None of the fetal urine analytes examined yielded clinically significant accuracy in predicting poor postnatal renal function. The 2 most accurate parameters were calcium level greater than 95th percentile for gestation (likelihood ratio [LR]+ 6.65 [0.23,190.96], LR− 0.19 [0.05,0.74]) and sodium level greater than 95th percentile for gestation (LR+ 4.46 [1.71,11.6]; LR− 0.39 [0.17,0.88]). With respect to ultrasonographic findings, the second systematic review and meta-analysis showed that abnormal renal cortical appearance (as defined as echogenicity and/or cystic changes) showed the best predictive accuracy (sensitivity of 0.57 [0.37,0.76], specificity of 0.84 [0.71,0.94], AUC of 0.78, and LR+ 1.29–9.10). The LR+ for severe oligohydramnios was moderately useful ranging from 3.63 to 7.00. Both of these reviews highlight the current limitations in the evidence available to support fetal intervention for LUTO.
Therapeutic options
Prenatal management of significant LUTO that compromises renal function and fetal urine production is predicated on establishing adequate drainage between the fetal bladder and the surrounding amniotic space. To date, the mainstay for fetal intervention for LUTO has involved urinary diversion via the placement of a vesicoamniotic shunt. Alternative techniques include open fetal surgery with fetal vesicostomy and/or pyelostomy, or endoscopic management with direct in utero valve ablation using fetal cystoscopy. The benefits of the latter would be avoiding the high complication rates of vesicoamniotic shunting and allowing the bladder to cycle and resume some semblance of normal development.
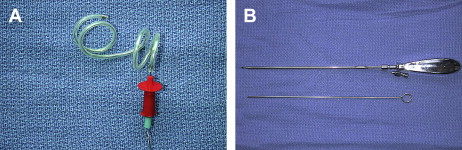
Vesicoamniotic shunting
Technical Considerations
Vesicoamniotic shunting is performed by placement of a specialized double pigtail shunt (Rocket Medical, Washington, UK and Cook Urological, Spencer, IN, USA) under ultrasound guidance between a distended fetal bladder and the surrounding amniotic space ( Fig. 1 ). Color-flow Doppler allows visualization of the umbilical arteries, which course laterally to the fetal bladder. Amnioinfusion is performed in the setting of oligohydramnios to allow for a potential space into which the intra-amniotic end of the catheter may be placed. Technical considerations involve low placement of the shunt into the bladder to prevent displacement following bladder decompression. The optimal site for trocar entry is midway between the pubic ramus and the insertion of the umbilical cord.