(1)
Department of Physiology and Biophysics, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216, USA
Keywords
PregnancyHypertensionAngiogenic factorsPlacentalKidneyVEGFImmune mechanismsNitric oxideEndothelinIntroduction
Preeclampsia (PE) is considered a pregnancy-specific syndrome that is diagnosed when new-onset hypertension and proteinuria occur after 20 weeks of gestation [1–3]. PE can progress rapidly to more severe complications such as seizures (eclampsia) and hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome, which can lead to cerebral hemorrhage, organ failure, and death [1–3]. PE is estimated to affect 5–7 % of all pregnancies. Despite being one of the leading causes of maternal death and a major contributor of maternal and perinatal morbidity, the mechanisms responsible for the pathogenesis of PE have not been fully elucidated. Hypertension associated with PE develops during pregnancy and remits after delivery, implicating the placenta as a central culprit in the pathogenic process [1–3]. PE affects the vasculature of many target organs including the brain, liver, and kidney [1–3]. Indeed, glomerular endotheliosis is considered an important characteristic lesion of women with PE [1–3].
Although numerous factors including genetic, behavioral, and environmental factors have been implicated in the pathogenesis of PE, an important initiating event for the development of PE is thought to be placental ischemia/hypoxia ([1–3]; see Fig. 13.1). The hypoxic placenta, in turn, releases a variety of soluble factors that have profound effects on the peripheral vasculature and arterial pressure regulation. These factors include a host of molecules such as the soluble vascular endothelial growth factor (VEGF) receptor-1 (sFlt-1) , the angiotensin II type 1 receptor autoantibody (AT1-AA), and inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), which in turn generate widespread dysfunction of the maternal vascular endothelium. This dysfunction manifests as enhanced formation of factors such as endothelin , reactive oxygen species (ROS), and augmented vascular sensitivity to angiotensin II. In addition, PE is also associated with decreased formation of vasodilators such as nitric oxide (NO) . These alterations in vascular function not only lead to hypertension but multiorgan dysfunction including the brain, kidneys, and liver as well. Since PE remains to be one of the leading causes of maternal death and perinatal morbidity, identifying the mechanisms underlying abnormal placentation and the factors that link placental hypoxia and maternal cardiovascular and renal abnormalities remain important areas of investigation.
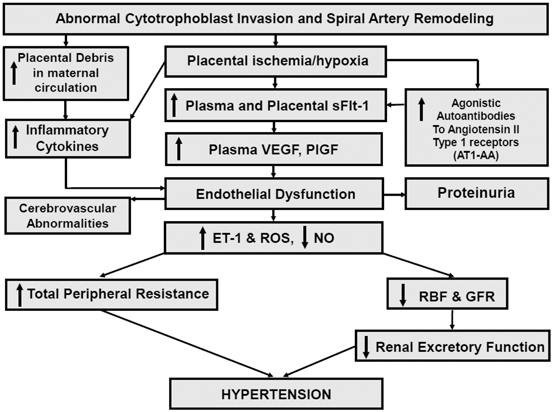
Fig. 13.1
Hypothetical scheme depicting how abnormal cytotrophoblast invasion and subsequent reductions in spiral artery remodeling results in endothelial dysfunction and hypertension in PE
Abnormal Spiral Artery Remodeling and Placental Hypoxia in PE
During normal pregnancy, fetally derived cytotrophoblasts migrate to the uterine tissue in an orchestrated manner to invade and remodel the maternal uterine spiral arteries to ensure adequate oxygen and nutrient delivery to the developing uteroplacental unit [1–5]. This complex migration and invasion process results in the conversion of the high-resistance, small-diameter spiral arteries into high-capacitance, low-resistance vessels [4, 5]. It is believed that poor cytotrophoblast migration and/or vascular invasion during PE, leads to abnormal spiral artery remodeling and inadequate oxygen delivery to the developing uteroplacental unit [4, 5].
While the exact mechanisms responsible for the abnormal placental trophoblast migration/invasion and vascular remodeling in PE are unclear, results from a recent study by Hunkapiller et al. found that the absence of Notch2 in mice is associated with reduced vessel diameter and placental perfusion [6]. Additional findings that perivascular and endovascular cytotrophoblast often fail to express the Notch ligand, JAG1, in PE provides further evidence that defects in Notch signaling may be important in the pathogenesis of this pregnancy syndrome . Another recently described molecular pathway implicated in placental vascular development is the transcription factor storkhead box 1 (STOX1), a member of the winged helix transcription factor family [7]. Transgenic overexpression of STOX1 in the mouse leads to a phenotype that mimics PE in several key ways, most notably an increase in systolic blood pressure during gestation and elevated maternal circulating levels of soluble fms-like tyrosine kinase (sFlt)-1 and soluble endoglin [7]. While data from these recent studies are intriguing, much work remains to be done to elucidate the role of factors in mediating abnormal spiral artery remodeling in PE.
Factors Linking Placental Ischemia/Hypoxia with the Maternal Hypertension
Angiogenic Factors
One of the most intensely studied pathways in the pathophysiology of PE is that related to VEGF signaling [1–3, 8–11]. VEGF and the placental growth factor (PlGF-1) are also critically important in the maintenance of proper endothelial cell function in adult animals [8–11]. The VEGF signaling pathway came to prominence with the discovery of elevated circulating and placental levels of the soluble form of the VEGF receptor, fms-related tyrosine kinases-1 (sFlt-1) in preeclamptic women, especially in late gestation [8–11]. sFlt-1 is a circulating soluble receptor for both VEGF and PlGF, which when increased in maternal plasma leads to less circulating free-VEGF and free-PlGF, thus preventing their availability to maintain maternal endothelial integrity. In the kidney, this inactivation of free VEGF is believed to cause endotheliosis and proteinuria [12]. Subsequent studies of the regulation of sFlt-1 in cell culture and placental tissue in vitro have demonstrated that sFlt-1 is released from placental villi and trophoblast cells in response to reduced oxygen tensions similar to that seen in an ischemic placenta [1–3]. While sFlt-1 production appears to be regulated by hypoxia inducible factor-1, other factors such as TNF and the agonistic autoantibody to the AT1-AA also appear to be involved [1–3, 13] .
Several lines of evidence support a role for angiogenic factors in the pathogenesis of hypertension during PE. Several clinical studies have reported that sFlt-1 levels are strongly correlated with the severity of the PE [8–11]. In addition, chronic intravenous administration or adenovirus delivery of sFlt-1 to pregnant rats, to mimic plasma concentrations of sFlt-1 observed in preeclamptic women, decreases free VEGF and PlGF and produces hypertension and proteinuria [9, 14]. Moreover, a promising pilot study recently demonstrated that sFlt-1 could be removed from the maternal circulation of preeclamptic women by apheresis safely, and that this therapy reduced both blood pressure and proteinuria, with a trend toward increased gestational duration [15].
In addition to playing a pathogenic role in PE, angiogenic factors have been proposed as diagnostic markers for the syndrome. Several clinical studies were designed over the past decade to determine the potential of angiogenic factors as prediction tests in PE [1, 11]. While their accuracy fell short of sensitivities and likelihood ratios required for clinical use, prediction was much more reliable for early-onset PE. Ohkuchi et al. recently found that the sFlt-1 to PlGF ratio was a useful component for the prediction of PE when measured at 26–31 weeks of gestation [16]. Likewise, Perni et al. examined angiogenic factors in patients who had preexisting hypertension with superimposed PE, and found higher circulating levels of sFlt-1 prior to the 20th week of gestation in these patients versus pregnant women who had preexisting hypertension but did not develop PE [17]. These studies, along with other recent work, suggest that angiogenic balance could be a reliable marker of PE and allow detection prior to the onset of patient symptoms [11, 18]. Rana and colleagues recently suggested that angiogenic proteins alone account for the disease’s major phenotypes and therefore are extremely specific for both diagnosis and prognosis [11]. They also suggested that future screening studies should focus on prediction of the angiogenic form of PE rather than disease diagnosis based on nonspecific clinical criteria [11].
Immune Factors and Inflammation
The pathophysiology of PE is also thought to involve immune system abnormalities and inflammation [18–20]. Redman and colleagues proposed that fragments shed from the placental surface include pro-inflammatory proteins that may contribute to the systemic inflammatory response in normal pregnancy and the exaggerated inflammatory response in PE [19]. Supporting this concept are findings that proinflammatory cytokines, such as IL-6 and TNF-α, are elevated in preeclamptic women and placental ischemic rat models [18]. Moreover, infusion of proinflammatory cytokines into pregnant animals produces significant elevations in blood pressure [3, 18].
Maternal immune tolerance mechanisms are also implicated in the pathophysiology of PE. This maternal immune tolerance involves crucial interactions between regulatory CD4+ T cells and uterine natural killer cells recognizing and accepting the fetal antigens and facilitating placental growth. Partial failure of this interaction is thought to lead to poor placentation and dysfunctional placental perfusion and chronic immune activation originating from the placenta. Preeclamptic women have a decrease in circulating regulatory CD4+ T cells. Moreover, placental ischemic rats have a 47 % decrease in regulatory CD4+ T cells in the peripheral circulation when compared to normal pregnant rats [19]. T helper 17 cells, which are upregulated in a variety of autoimmune disorders, are also increased in preeclamptic women, and in placental ischemic rats [20]. While these data support the hypothesis that hypertension in response to placental ischemia represents a shift from the normal anti-inflammatory state of pregnancy to a pro-inflammatory state, the quantitative importance of CD4+ T cells and T helper 17 cells in the pathophysiology of PE remains to be determined [18].
A number of recent studies have also indicated that women with PE produce a novel agonistic autoantibody to the angiotensin II type I receptor [20–23]. Dechend and colleagues reported that sera from preeclamptic women contain an IgG (type 3) autoantibody that reacts with the AT1 receptor [22]. The binding of the AT1-AA to the seven amino acid stretch of the second extracellular loop of the angiotensin II type 1 receptor stimulates a chronotropic response from rat neonatal cardiomyocytes which can be attenuated with administration of an AT1 receptor antagonist. The is the basis of the bioassay primarily used for the detection of the autoantibody . These autoantibodies, isolated over a decade ago in preeclamptic women, have been studied more intensively recently, including their identification in the circulation of rats undergoing placental ischemia [3, 18, 24]. While infusion of the AT1-AA directly into pregnant animals results in moderate hypertension, the pathogenic importance of these antibodies remains to be fully elucidated, as their presence has been noted postpartum in a subset of preeclamptic patients even after the symptoms were resolved. Further studies are needed including determining how these unique antibodies are produced and how they interact with the other pathogenic agents in PE to produce the clinical phenotype .
Endothelin
There is growing evidence to suggest an important role for endothelin-1 (ET-1) in the pathophysiology of PE [25, 26]. Multiple studies have examined circulating levels of ET-1 in normal pregnant and preeclamptic cohorts, and found elevated levels of plasma ET-1 in the preeclamptic group, with some studies indicating that the level of circulating ET-1 correlates with the severity of the disease symptoms, though this is not a universal finding [25]. ET-1, however, is produced locally and plasma levels typically do not reflect tissue levels of the peptide. Animal studies have shown that a myriad of experimental models of PE (placental ischemia, sFlt-1 infusion, TNF-α infusion, and AT1-AA infusion) are associated with elevated tissue levels of ET-1 [2, 3, 25, 26]. A recent report also indicated increased vascular contractility to big ET-1 in the reduced uteroplacental perfusion pressure rat model of PE, an effect that was attributed to a greater contribution of matrix metalloproteinases to cleave bET-1 to ET-1 [27]. Finally, the fact that hypertension in pregnant rats, induced by placental ischemia or chronic infusion of sFlt-1, TNF-α, or AT1-AA [25, 26] can be completely attenuated by ETA receptor antagonism, strongly suggests that ET-1 is a final common pathway linking factors produced during placental ischemia to elevations in maternal blood pressure.
Nitric Oxide
Studies have suggested important roles for NO as a regulator of arterial pressure under various physiological and pathophysiological conditions [28–30]. NO is synthesized endogenously from L-arginine, oxygen, and nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) by various NO synthase (NOS) enzymes. NO production is elevated in normal pregnancy and these increments appear to play an important role in the vasodilatation that occurs in healthy pregnancy [28–30]. Thus, it was postulated that NO deficiency during PE might be involved in the disease process. Whether there is a reduction in NO production during PE is controversial. Much of the uncertainty originates from the difficulty in directly assessing the activity of the NO system in a clinical setting. Assessment of whole body NO production via measurement of 24-hour nitrate/nitrite excretion has yielded variable results, likely due to difficulties in controlling for factors such as nitrate intake and excretion [2–3]. Thus, the relative importance of NO deficiency in the pathogenesis of PE has yet to be fully elucidated.
In support of a role for NO deficiency in the pathogenesis of PE are reports from several laboratories that chronic NOS inhibition in pregnant rats produces hypertension associated with peripheral and renal vasoconstriction, proteinuria, intrauterine growth restriction, and increased fetal morbidity, a pattern resembling the findings of PE [28–30]. Placental ischemia has been reported to result in endothelial dysfunction and reduced NO production in some but not all vascular beds [29]. Moreover, L-arginine supplementation in animal models and in women with PE reduces blood pressure and improves pregnancy outcomes in some but not all studies [29]. Finally, hypertension induced by sFlt-1 in pregnant animal models is associated with significant reductions in NO synthesis [14].
Endoplasmic Reticulum and Oxidative Stress
Endoplasmic reticulum stress activates a number of signaling pathways aimed at restoring homeostasis. Burton and colleagues proposed that this mechanism to restore homeostasis fails and apoptotic pathways are activated to alter placental function in women who develop PE [31]. In addition, chronic, low levels of endoplasmic reticulum stress during the second and third trimesters may result in a growth-restricted phenotype. They also propose that higher levels of endoplasmic reticulum stress lead to activation of pro-inflammatory pathways that may contribute to maternal endothelial cell activation [31]. While endoplasmic reticulum stress is known to occur in PE, the importance of this abnormality in the pathophysiology has yet to be fully elucidated.
Oxidative stress has also been implicated in PE, as increased concentration of several oxidative stress markers have been reported systemically in preeclamptic women, among these peroxynitrite [32, 33]. Peroxynitrite concentrations in vascular endothelium were much higher in preeclamptic women versus normal gestation, concurrent with decreased levels of superoxide disumutase (SOD) and NOS [32, 33]. There is also evidence of increased oxidative stress during gestation in the placental ischemic rat hypertensive model, suggesting a link between placental ischemia/hypoxia and the production of reactive oxygen species [2, 3]. For example, the SOD-mimetic drug tempol, led to significant attenuation of the hypertensive response [2, 3]. In a related study, administration of the NADPH oxidase inhibitor apocynin also significantly attenuated placental ischemia-induced gestational hypertension, implicating the enzyme as an important source of pathogenic ROS in the reduced uterine perfusion pressure (RUPP) animal [2, 3]. Failure of the drug to fully normalize blood pressure, however, leaves open the possibility that alternative ROS production pathways are at work in the RUPP model. Further studies into the mechanism of ROS production in animal models of PE should help shed light into the importance of oxidative stress in the pathophysiology of PE and perhaps allow the identification of useful antioxidant strategies. It remains to be seen whether ROS production is a primary or secondary cause of PE pathophysiology, and how effective manipulation of the system will be in the search for effective therapies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






