A “biomarker” (biological marker) is an indicator of a bodily function that can be objectively measured. A wide range of possible biomarkers for IBS have been considered but at present only gut transit measured using radio-isotope markers meet the criteria of reproducibility and availability. While barostat studies perform reasonably in expert centers, to do them reproducibly requires considerable effort and standardization. This makes them unsuitable for widespread use. However radio-isotope tests are expensive and of limited availability so the search for other more convenient markers including blood and stool tests is still an important goal for the future.
What is a biomarker?
A biomarker (biological marker) is an indicator of a physiological or pathological state that can be objectively measured and evaluated. It contrasts with subjective patient reported outcomes by usually having lower variability and therefore greater power to detect effect of treatments or differences between groups. A good example of a biomarker used in other fields is the forced expiratory volume in 1 second (FEV 1 ). This biomarker is an objective measure of respiratory function that assesses airway resistance, which is a critical feature of asthma. It is simple, inexpensive, and reproducible. The standard is to use the best of 3 to allow for learning of the technique. It has been widely used and shown to be reproducible, responsive to treatment, and also to indicate the state of the disease. In pulmonology other measures are also used to study asthma, including mucosal histology obtained from bronchial biopsy, sputum eosinophil counts and sputum culture. The advantages of the FEV 1 when compared with these other more invasive and technically demanding tests are its noninvasiveness, patient acceptability, and its responsiveness to many treatments. It is also apparent that the value of any particular biomarker will depend on the treatment being assessed; for example, if an antibiotic is being tested in infective exacerbation of asthma, then sputum culture might prove more valuable in predicting response.
Criteria for a Useful Biomarker in Irritable Bowel Syndrome
A useful biomarker for irritable bowel syndrome (IBS) would be simple and easy to use. It would be patient acceptable and reproducible (low intrasubject variability). Ideally it should also have low intersubject variability within defined groups. This variability is important because it reduces the number needed to test (NN test) to detect a minimally important difference with an acceptable power, which is particularly important when evaluating new treatments to allow a rapid decision on whether to pursue development or move on to another molecule. Because most studies are done in multiple centers, it is important that the technique of measurement should be readily transferable between centers and, therefore, should not need highly sophisticated equipment or require scarce expertise. Finally, all these things are of little value if the technique is too expensive.
Pathophysiology of IBS relevant to choosing a biomarker
IBS patients are a highly heterogeneous group because the symptoms are quite nonspecific and may reflect numerous pathophysiologies, including altered transit and sensitivity. For the purposes of this article, it is sufficient to say that most authors agree that in any one patient a range of factors, including stress, somatization, anxiety, neuroticism, diet, and prior infection, contribute to symptoms mediating both altered visceral sensitivity and disturbances of bowel transit. Biomarkers that could identify the main mechanism in each individual patient would be of undoubted value.
The other important consideration in using biomarkers to predict or evaluate response to a new therapy is that to be useful, the biomarker should relate to the mode of action of the drug being evaluated. Many recently introduced drugs have altered gastrointestinal (GI) transit, including prokinetics, such as tegaserod, prucalopride, and velusetrag ; and secretagogues aimed at treating constipation, including lubiprostone and linaclotide. Others delay transit, such as the 5HT3 receptor antagonists (alosetron and ondansetron ), or reduce visceral hypersensitivity, such as amitriptyline and the 2,3-benzodiazepine receptor agonist dextofisopam. Although still in development, anti-inflammatory agents, such as mesalazine, have been shown to reduce mast cell numbers or to inhibit release of mast cell mediators, such as ketotifen. Since one of the main purposes of biomarkers is to identify subjects for which a specific treatment may be more appropriate, the majority of this article addresses measures of transit, visceral hypersensitivity, abnormal stress responsiveness, and inflammation. The author also considers newer proposed biomarkers that still require validation but might be useful in the future.
As Table 1 shows, there are many potential biomarkers reflecting different aspects of the pathophysiology of IBS. These biomarkers focus on changes in gut function and the associated microbiota. Of course, it is well recognized that there are important influences of the brain on the gut in IBS but as yet apart from psychometric assessments based on patient reports, the complexity of the brain has defied the development of simple biomarkers. Although differences in brain activation in response to painful stimuli between subjects with IBS and controls can be demonstrated using positron emission tomography scanning or fMRI, the techniques are difficult and results variable between centers. Furthermore, the equipment is extremely expensive, therefore, patterns of brain activation by peripheral stimuli do not meet our criteria for a good biomarker.
| Mechanism | Potential Biomarkers |
|---|---|
| Disordered motility/secretion leading to altered transit and altered stool form | Manometry/transit tests/stool charts |
| Visceral hypersensitivity | Rectal barostat/cutaneous stimulation |
| Abnormal autonomic reactivity | Heart rate variability/response to pain |
| Stress response | Cortisol/response to visceral stimulus |
| Mucosal inflammation | Mucosal biopsy |
| Evidence of immune activation | Serum/peripheral blood mononuclear cells cytokine production |
| Increase fecal proteases | Stool test |
| Altered gut flora | Stool DNA/culture/bacterial metabolite assessment |
| Food allergy | Skin prick test, serum antibodies |
| Genetic polymorphisms | Single-nucleotide polymorphism assays |
Link Between Biomarker and Clinical Response
Although in the end a treatment has to improve patients’ quality of life and how they perceive their symptoms, such measures are always influenced by many factors other than the local GI ones. Thus, all stimuli coming from the gut have to be interpreted in light of previous experiences, current emotional state, and psychological stressors before they are converted to symptom reports. This means that an intervention or drug that alters gut function may have a weak effect on patient-reported outcomes and the size of the clinical trial needed to show the effect is likely to be large, on the order of many hundreds.
Biomarkers as Surrogate Endpoints
By restricting the end points to a biomarker closer to the gut function, variability should be less and hence standardized effect sizes should be larger and the number of individuals needed to test to show an effect much less. This is of considerable value in the development of new drugs. By being more focused and less holistic, these measures are much more likely to be culture independent and hence easier to translate across multiple centers and countries, again a value feature in drug development that is often multinational. A major use of biomarkers is therefore in mode of action or proof of concept studies during phase I and early phase II. Although on its own not acceptable as the only outcome measure in phase III studies, biomarkers might also be valuable as entry criteria to improve responder rates or as secondary end points.
Assessing gut motility
Motor patterns are important in determining transit and also because they may cause pain, a major symptom in IBS that substantially determines perceived severity. The gut is insensitive to many stimuli, including thermal and chemical injury, but is responsive to distension and powerful contractions. Several studies have clearly linked powerful colonic contractions with abdominal pain ( Fig. 1 ).
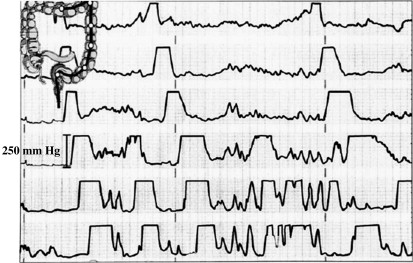
Although these contractions are crucial to the pain, as a biomarker, manometry’s major problem is the requirement for intestinal intubation. The original studies were done using naso-intestinal intubation, which is extremely arduous, taking several days to properly position the manometry catheter. These uncomfortable experiments have mainly been done with paid volunteers. The number of patient studies is limited and the subjects studied are likely to be a highly selected sample. Furthermore, the technique is expensive and technically demanding and hence impracticable for use as a biomarker. More recently, the technique of retrograde cannulation of the colon at colonoscopy allowing the positioning of probes, which are clipped to the mucosa, has been developed. This technique is much quicker and more patient acceptable but still highly invasive and expensive. Although useful perhaps for the early stages of proof-of-principal concepts, such techniques are unlikely to win widespread use in clinical trials or in clinical practice.
Even if such techniques were patient-acceptable, measures such as the duodenal motility index, although reasonably reproducible, show substantial intersubject variability in normal subjects with a coefficient of variation (CoV) (standard deviation divided by mean) of 13351/30708 (43%), giving a total NN test of 96 (48 per group) to show a 25% change in mean index with a power of 80% in a random controlled trial (RCT) of drug against placebo.
Recordings in the colon show a highly erratic pattern requiring long-term recordings to give meaningful results, which has only been done in a few studies. However, Clemens studied 12 subjects with IBS and reported a CoV of sigmoid motility over 24 hours of just 7%, indicating this would require only 3 subjects per group in a parallel group design, a total of 6 to detect a 25% change with 80% power if they could be persuaded to undergo 24-hour recordings. Almost identical figures were found by Rao in healthy women with a CoV of 7% and just 5% in subjects who were constipated. Alternative measures might be a frequency of specialized propagating pressure waves assessed over 24 hours with an indwelling rectal probe that gives a NN test of approximately 18. Although attractive for proof-of-principle studies, 24-hour intubation is too demanding on subject and investigator to be practical for large clinical studies.
The barostat can be used to assess motility and also tone and compliance. The performance characteristics of colon in tone and motility indices have been recently reported. The intersubject CoV was 22.8% for colonic compliance, 30.8% for fasting tone, and 35.9% for tone 30 minutes postprandially. This finding gave a NN test of 28, 25, and 28, respectively.
Wireless motility-pH capsule
Compared with manometry or a barostat study requiring intubation, this wireless pH, pressure and temperature sensitive capsule has obvious appeal. At 28.8 mm long by 11.7 mm in diameter, it is somewhat larger than a normal tablet but still readily swallowed by most patients. There is none of the pharyngeal discomfort associated with prolonged manometry and the patients are freely mobile. The temperature, pressure, and pH profile enable it to be tracked through the intestine with the sharp rise in pH that occurs on passing from the stomach to the duodenum and the sharp fall in temperature that occurs when the capsule is expelled readily identifying gastric, small bowel, and colonic transit. The rate of transit correlates well with the standard radio-opaque markers, although both show wide variability. The pressure tracings can also be used to derive a motility index based on the area under the curve of the pressure versus time ( Fig. 2 ) however, the clinical utility of this data has not been established. However, the intersubject CoV is high: 88% in constipated IBS and 127% in healthy volunteers. With these values, the NN test to show a 25% change in motility using a parallel group design would be a prohibitive 195 subjects per group.
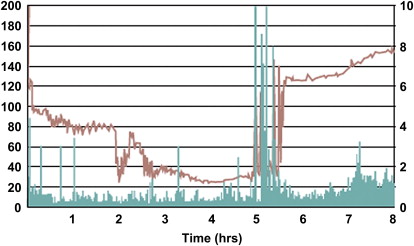
Transit as a surrogate for motility
In contrast to manometry, following the transit of a radioisotopically labeled meal through the gut is highly patient acceptable, can be done with low doses, and yields measures that have an acceptably low day-to-day variability while separating out important patient groups. The earliest techniques of measuring whole gut transit incorporated daily ingestion of radio opaque pellets on days 1 to 3 with a plain radiograph at day 4. Transit in hours through the colon was estimated to be 1.2 times the number remaining. The normal range of such a measure is substantial with healthy individuals having transits that vary from 6 to 64 hours. The coefficient of variance in women was 46%, giving a total NN test of 108 using a parallel group design, although this number falls to just 29 using a crossover design. Despite this variability, this method has been widely used and shown differences between patient groups, such as slow transit in those with constipated IBS. Furthermore, colonic transit measured by this method is responsive to therapeutic interventions, such as the prokinetic renzapride or the effect of bowel cleansing in constipation, although these studies used a crossover design that, by using each subject as their own control, reduces the numbers needed to test substantially. Mean colonic transit of markers measured in this way correlates well with geometric center of the pellets (r = 0.88) and with stool consistency ( Fig. 3 ).
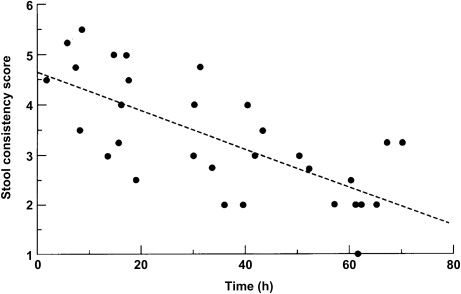
The reproducibility of median stool form in 2 28-day periods was tested by Degen and colleagues and shown to have an intrasubject CoV of repeated measures of approximately 33%. Earlier studies have shown an intersubject CoV of 42% giving a NN test of 90 (45 per group). However, it is easily assessed and in large clinical studies this measure shows the clinical efficacy of alosetron.
The pressure-sensitive radio pill can also be used to measure transit, although it has high intersubject variability being a single estimate of transit of one particle rather than a weighted average of many, like scintigraphy or radio-opaque pellets. In a recent study the mean transit time for constipated subjects was 46.7 (24.0–91.9) hours. This finding gives a CoV of 36% and a total NN test of 68 to detect a 25% change in transit time using a parallel group design, although this falls to just 18 if a crossover design is used.
Transit by scintigraphy
By contrast, with the limited use of manometry the more patient-friendly scintigraphic tests of transit have been used extensively in drug evaluation. Although being used initially to assess both gastric emptying and small bowel transit, the majority of the work in irritable bowel syndrome has focused on colonic transit. Following the first description, the technique was applied to demonstrate delayed transit in constipation and its acceleration by a prokinetic cisapride. The initial technique involved a laborious cecal intubation and was soon replaced by the use of enteric-coated pellets designed to release the radioactive marker in the ascending colon without the need for intubation. Further studies with the whole-gut transit method established that scanning at just 2, 6, and 24 hours was able to identify disordered gastric emptying and accelerated intestinal transit at minimum cost. Subsequent studies used this technique to demonstrate the efficacy of alosetron in subjects with carcinoid diarrhea, the acceleration of oral cecal transit by tegaserod in constipation-predominant IBS, and the effects of renzapride, lubiprostone, and linaclotide.
Early studies suggested that the half clearance time of the ascending colon was an important predictor of stool form. However, a recent study showed that the intersubject CoV was 40.9%, so the NN test would be 82 in a parallel group design. The performance characteristics of the scintigraphic assessment of gut transit have been assessed looking at the reproducibility and intersubject variability in healthy volunteers ( Table 2 ). The clinically important effect sizes based on previous studies were considered to be 25% different in gastric residual at 4 hours and a 1.5 difference in geometric center at 48 hours. Certainly for the colonic transit measurement, the geometric center at 48 hours requires acceptably small NN test (14 per group) for the detection of a 25% difference with 80% power using the standard parallel group design.
| n | GE t1/2 | Colon Filling 6 h | GC 24 h | GC 48 h | |
|---|---|---|---|---|---|
| CoV intersubject | 21 | 30.4 | 30 | 37 | 24 |
| CoV intrasubject | 21 | 14 | 19 | 28 | 14 |
| NN test to detect a 25% difference | — | 48 | 48 | 70 | 30 |
| NN test a | — | 82 | 96 | 46 | 28 |
a NN test is the number needed to test in an RCT of active versus placebo to detect clinically significant differences with 80% power.
However, abnormalities of transit are not a uniform feature of irritable bowel syndrome and in a recent, large study of 118 subjects with IBS, abnormal transit was only documented in 38 subjects, the remaining subjects showed a mixture of rectal hypersensitivity (25), hyposensitivity (20), and no abnormality in transit or sensitivity (39) ; therefore, it is apparent that other measures are needed.
Visceral sensitivity
Visceral hypersensitivity has been assessed over the last 15 years using the rectal barostat as a reproducible, ethically approved method of inducing abdominal pain without tissue injury. Although early studies suggested that 61% had an abnormally low pressure threshold for pain, more recent studies have reported much lower incidence; the recent series from the Mayo Clinic reported only 7.6% having thresholds for pain sensation below the 10th percentile and 13% having thresholds above the 90th percentile. Recent studies using decision theory analysis suggest that neurosensory sensitivity in IBS was no different from healthy controls and that the lower pain thresholds were a reflection of a greater tendency to report pain for any stimulus. Although the reproducibility of the threshold for pain in the short term is reasonable, with intrasubject CoV being 23%, this is not true for other sensations, such as urgency and discomfort with CoV of 41% and 47%, respectively. The same study showed that similar protocols performed at different centers produced similar results and that a 25% difference between 2 study groups could be demonstrated in a parallel group design for pain threshold using 14 individuals per group, a total of 28 in an RCT. Other endpoints, such as urgency and discomfort, would require much larger numbers, but a crossover design reduced the numbers substantially to just 6 per group. By contrast, the more subjective sensory rating for pain at 36 mmHg distension gave a CoV of 56% and number needed to test of 80 per group.
Despite reasonable reproducibility in the short term, the validity of pain thresholds as a measure of drug efficacy remains a problem for using this as a biomarker because the link between the reported thresholds and symptoms is weak. A longitudinal study performed over a period of 12 months with testing every 3 months showed that despite relative constant symptom severity the discomfort threshold in subjects with IBS moved into the normal range when the study was repeated, suggesting that part of the low thresholds in IBS relate to the stress of the first experience of the testing situation and that this may wear off with time.
Although barostats have been developed for use in animals and widely used to screen drugs, the link between the animal data and subsequent success or failure in clinical trials has been poor. As Table 3 shows, drugs that increased barostat pain thresholds, such as asimadoline and fedotozine, failed in large clinical trials; whereas, drugs that do not alter the threshold, such as lubiprostone and citalopram, have been successful. There is also the striking example of the tachykinin antagonists that are effective in animal models but that appear to have failed in clinical trials that have been completed some years ago but never published. Likewise, asimadoline, a kappa opioid agonist, was effective in decreasing sensation during rectal distension and decreased postprandial fullness during ingestion to maximum satiety of a liquid test meal. However, in clinical trials it failed to achieve adequate relief of IBS pain compared to placebo and failed to improve symptoms in functional dyspepsia. Subgroup analysis in the IBS trial showed that in diarrhea-predominant IBS with at least moderate pain at baseline, one of the 3 doses of asimadoline did produce improvement in the total number of months with adequate relief. However, being an unplanned subgroup analysis, this may well represent a chance finding.
| Drug | Increase in Barostat Pain Threshold | Study | Effectiveness in Clinical Trial | Study/Meta-analysis |
|---|---|---|---|---|
| Ondansetron | No (compliance increased) | Zighelboim et al 1995 | Yes | — |
| Alosetron | No (compliance increased) | Delvaux et al 1998 | Yes | Cremonini |
| Fedotozine | Yes | Delvaux et al 1999 | No | Lembo 2006 |
| Hypnosis | No | Palsson et al 2002 | Yes | Palsson et al 2002 |
| Fluoxetine | No | Kuiken 2003 | No | — |
| Citalopram | No | Tack et al 2006 | Yes | Tack et al 2006 |
| Talnetant | No | Houghton et al 2007 | No | Unpublished www.Clinicaltrials.gov , registered 2005 |
| Lubiprostone | No | Sweetser et al 2009 | Yes | Drossman et al 2009 |
| Saredutant | Yes in animal models | Toulouse et al | ? No | Trials unpublished, 2006 |
| Asimadoline | Yes | Delgard-Aros et al Delvaux et al | No in IBS or functional dyspepsia | Mangel et al 2008 Talley et al 2008 |
| Amitriptyline | Yes | — | Yes | — |
| Clonidine | Yes | Bharucha et al 1997 Viramontes et al 2001 Camilleri et al 2009 | Effective but drowsiness excessive | Camilleri et al 2003 |
Clonidine stands out as being the exception in that it not only relaxes the rectum but also increases the threshold for pain and was effective in a small clinical trial, but unfortunately drowsiness limited its use.
The conclusion of this experience must be that although the rectal barostat can be used to demonstrate changes in visceral hypersensitivity, the link between this observation and changes in clinical symptoms is weak and should not be used as a basis for decision to proceed with development of a new product. Furthermore, as already alluded to, the barostat technique is difficult to standardize and technically demanding. It is thus unsuitable for use as entry criteria for a clinical trial or indeed to use in clinical practice.
Autonomic reactivity
Many patients think that stress aggravates their symptoms and several recent studies have demonstrated enhanced hypothalamic-pituitary axis (HPA) response. Patients with IBS-D show increased cortisol response after a meal and an increase in the low-frequency/high-frequency band ratio of heart rate variability. This ratio is thought to represent the balance between para sympathetic and sympathetic tone. Overall, women with IBS show no differences between controls and IBS, but when analysis is restricted to those with severe symptoms, parasympathetic tone appears lower in the constipated group compared with the diarrhea group. Small studies have reported differences in other cardiovascular parameters including a higher resting heart rate in IBS. Other measures, including cortisol response to the invasive procedure of sigmoidoscopy, did separate subjects with IBS from healthy controls and was higher in those with early life trauma regardless of whether they had IBS or not. The data from these trials suggests that salivary cortisol at baseline shows substantial variability and does not distinguish IBS from controls and would therefore not be a useful biomarker. Although the response to sigmoidoscopy showed clear-cut differences, it is not a practical test to be used in large numbers of subjects.
Markers of mucosal inflammation
The recognition that IBS can develop after a bout of gastroenteritis and the demonstration of evidence of immune activation in the mucosa has stimulated examination of mucosal biopsies using a range of techniques, including quantitative histology, mRNA for cytokines, and assessment of mediators released into the supernatant of cultured biopsies. Although there are indeed differences in mean cell counts between healthy controls and subjects with IBS, there is also considerable overlap. Furthermore, quantitative histology is demanding and highly dependent on technical expertise. Reproducibility has not been demonstrated, although recently evidence of responsiveness to treatment has been forthcoming. A recent randomized control trial of mesalazine against placebo did show a significant fall in mast cell numbers. Thus, in mechanistic studies particularly using anti-inflammatory drugs, mast cell number could act as a biomarker both for entry into the study and as an endpoint. In that study, the placebo arm can be regarded as a reproducibility study with a CoV of approximately 20% and a number needed to test of 22, which is at least as good as other biomarkers. Further studies in this area would be valuable.
Several studies have reported on mediator release from incubated biopsies. Although of great mechanistic interest, the variability in the biopsies is substantial and, without further standardization of the procedure, unsuitable as a biomarker. Whether this will change in the future with, for example, serotonin release correlating with response to serotonin antagonists remains to be established. In addition, there have also been several studies demonstrating increased cytokine production by peripheral blood mononuclear cells. Again, the variability is substantial and these have not been shown to be responsive to treatments and do not seem ready to become biomarkers at present.
Stool markers of mucosal inflammation
Looking at markers released by neutrophils into the stool can assess inflammation in the gut. These markers are not a feature of irritable bowel syndrome where the mucosal changes are restricted to chronic inflammatory cells, such as lymphocytes and macrophages. The best-validated test is fecal calprotectin, a molecule resistant to bacterial degradation released by mucosal polymorphonuclear white blood cells with sensitivity for inflammatory bowel disease of 89% and a specificity of 79%. Therefore, a negative fecal calprotectin increases the risk of IBS but does not exclude inflammatory bowel disease because approximately 11% have normal values. Negative biomarkers are less useful than positive ones because they cannot be used to evaluate responses. In this respect, β defensin is interesting because, unlike calprotectin, it shows an increase in IBS. However, variability is high and the intersubject CoV is substantial with a mean value of 76.0 ± 67.9, giving a CoV of 89% and requiring 400 subjects to detect a 25% difference with an 80% power.
Fecal proteases
Another potential biomarker recently described is the fecal protease. These functional assessments of enzyme activity detect serine proteases within the gut lumen, which are likely to be mainly of endogenous origin but also include possible bacterial enzymes. Recent reports indicate that these are elevated specifically in IBS-D. The importance of this finding relates to the demonstration that there are protease activated receptors type 2 on epithelial cells and enteric nerves with activation that increases gut permeability and induces inflammation. The fecal supernatants from subjects with IBS containing these serine protease were able to increase visceral hypersensitivity in a rat barostat model. However, the values of trypsin units per milligram of protein were 2079 with a standard deviation of 1860, which gives a CoV of 89% and a number needed to test of 400 to detect a 25% difference with an 80% power in a parallel group design. Plainly, greater standardization of stool collection and assay methods will be needed before such a marker could be useful.
Assessment of gut flora
There has been a revolution in the approach to understanding the gut flora brought about by the development of new DNA-based assessments. These assessments reveal that nearly two-thirds of the bacteria cannot be cultured, indicating that the picture we have been obtaining so far has been incomplete. The new technologies include density gradient gel electrophoresis of DNA generated by polymerase chain reaction (PCR) of the 16S ribosomal RNA gene and the more sophisticated human intestinal tract chip (HITChip), a phylogenetic microarray which uses nanotechnology to PCR more than 5000 markers to characterize around 1000 bacterial species. These new techniques have revealed a previously unrecognized complexity. Studies so far in IBS have indicated important differences but also great variability. Patterns are starting to emerge with evidence of imbalance in the different microbial communities, particularly in IBS-D. One consistent feature appears to be a decrease in overall diversity and the number of aerobic bacteria with an increase in aerobes, including Proteobacteria. Similar features are seen after infection and in inflammatory bowel disease. These changes in gut microbiota may well prove to be useful biomarkers for studies of treatments likely to alter the flora, such as probiotics and antibiotics, but like some of the other more experimental techniques discussed, this will take a lot more work before it can be applied in clinical practice.
Serum antibodies
Reports of increased incidence of antibodies to common food antigens have been made for more than 2 decades but they lack consistency. A recent report showed an increase in antibodies to flagellin antigens A4Fla-2 and A4Fla-X in 29% and 25% of subjects with IBS versus 7% in controls. However, similar increases were seen in Crohn disease, so this is not helpful diagnostically and antibodies rarely change rapidly in response to treatment, making this of little value on its own as a biomarker. There is already on the market the Prometheus serological panel, which combines several tests that detect the presence of inflammatory bowel disease anti- Saccharomyces cerevisiae antibody (ASCA), periplasmic antineutrophil cytoplasmic antibody (pANCA), and celiac disease (antihuman tissue transglutaminase [tTG]) together with some other antibodies in an unpublished algorithm to predict whether or not patients have IBS. Of the 10 antibodies examined, the ASCA, pANCA, and tTG are negative predictors ; whereas, the other antibodies, including antibodies against bacterial flagellin, tumor necrosis factor-α, and interleukin-1β, may be measures of previous infection and ongoing inflammation. Other proteins examined include Gro-α, a chemokine associated with chemotaxis, and a tissue inhibitor of metalloproteinase (TIMP-1), which may well be raised in inflammatory bowel disease. The sensitivity overall was only 50% with a specificity of 88%. The positive-likelihood ratio of 4.2 is in fact rather similar to other clinical criteria, such as the Kruis criteria published in 1984. Whether this can be improved on by adding different serum-based markers remains to be determined.
Skin prick tests and tests of food allergy
Although patients attending the immunology department with potential food allergy certainly share features in common with IBS, including abdominal pain and diarrhea, the true incidence of food allergy in patients attending IBS clinics seems fairly low with about 14.0% suspected and only 3.2% confirmed by objective testing. Patients who present with classic food allergy, including diarrhea and urticaria, after ingesting offending food stuffs are rarely misdiagnosed as IBS and usually see immunologists rather than gastroenterologists. Those without these features rarely turn out to have true food allergy when assessed by the gold standard, which is a double blind, placebo-controlled food challenge. More careful attention to symptoms suggests that the presence of frequent lower abdominal pain relieved by defecation and associated with bloating can distinguish IBS from food allergy. There are several poorly validated commercial tests that examine immunoglobulin G (IgG) antibodies for food that is extremely common within the normal population. One study showed IBS had significantly high IgG4 titres against wheat, beef, pork, and lamb compared with controls. Unfortunately these are not consistent in other studies that report increased titres to mostly different protein, although wheat was positive in both studies. One placebo-controlled trial did demonstrate a small benefit of a food-elimination diet based on IgG antibodies, but the majority of individuals were treated by means of wheat and milk exclusion so it unclear to what extent the antibodies are useful in predicting the correct diet. One earlier study reported that the presence of a positive skin prick test to certain foods increased the chances of responding to sodium cromoglycate, but this has never been repeated and in clinical practice skin prick testing in patients with IBS proves of little value.
Genetic polymorphisms
A genetic component of IBS is likely to be similar to that of many complex diseases with many genes providing small effects. The studies that have yielded information of the greatest value in gastroenterology have been performed in Crohn disease using more than 12,000 individuals to identify more than 30 genetic polymorphisms each associated with small (1.2) relative risks. By contrast, most IBS patient studies have less than 250 subjects and so it is not surprising that many of the findings are not reproducible. There are a small number of studies that suggest that genetic polymorphisms might predict drug responsiveness based on polymorphisms in drug metabolism enzymes and also the serotonin transporters gene. Thus, the homozygote ll for the SERT promoter polymorphism was associated with greater response to the 5HT3 receptor antagonist than the heterozygote sl . Likewise, the homozygote ll responded less well to the prokinetic 5HT4 receptor agonist, tegaserod. However, these studies were small and require replication in larger studies before they can be considered potential biomarkers of drug responsiveness.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



