Risk factor
Prevention
Treatment
Patient
Male gender
–
–
Comorbidities (pulmonary)
(Prehabilitation)
(Early mobilization, physiotherapy)
Procedure
New ostomy
–
–
Surgical approach
(Minimally invasive surgery)
–
Emergency operation
–
–
Operation time
Consider pre-emptive conversion
–
Blood loss/transfusion
(Excellent surgical technique)
–
Perioperative care
Fasting
No fasting
Early food intake
Fluid overload
Zero fluid balance
Carbohydrates
No bowel preparation
Early oral intake
Early discontinuation of IV fluids
PONV
No nitrous oxide
Short-acting anesthetics
PONV prophylaxis
5HT3 antagonist
Opioid treatment
Opioid-sparing strategy
Opioid-sparing strategy
Immobilization
Prehabilitation
Omission of drains, NG tubes
Early removal/omission of urinary catheter
Early mobilization
Pharmacological agents
Laxatives
?
+
Chewing gum
?
+
Opioid-sparing analgesia
+
–
Lidocaine
+
–
Alvimopan
+
–
Neostigmine
–
?
WSCA
–
+
Perioperative management has an important impact on POI, and can be either a major risk factor for POI or protect against it (Table 12.1). Perioperative fluid management shall serve as one example. Administration of excess perioperative intravenous fluid, especially saline, has a profound pathological effect on intestinal physiology [15]. Common pathophysiological features are intestinal edema, acidosis, and increased abdominal pressure resulting in wound healing problems, anastomotic complications, and POI (Fig. 12.1). Many different efforts have been undertaken over the recent years to shift the paradigm from POI being an inevitable part of abdominal surgery towards instead thinking of POI as a “preventable event” [16], as will be outlined in this chapter.
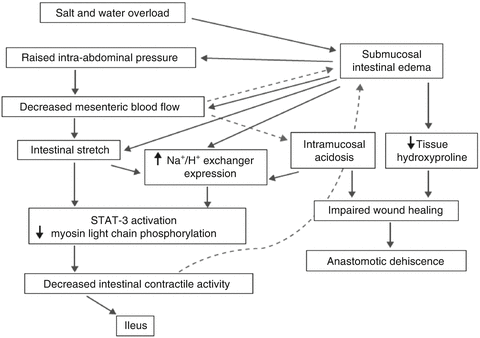

Fig. 12.1.
Pathophysiology of postoperative ileus related to salt and water overload. The effects of the physiologic stress response to surgical trauma can be exacerbated by the approach to perioperative care. When excessive saline is administered, overloading the patient with salt and fluids, intestinal edema and an increase of the intra-abdominal pressure result, contributing to the occurrence of postoperative ileus.
Prevention of Postoperative Ileus
1.
Early oral feeding. Malnutrition affects up to 30–40 % of patients undergoing major surgery. It is perhaps the most important potentially modifiable risk factor for morbidity and for infectious complications in particular. Nutritional interventions have proven effective to correct this risk constellation and thus to improve surgical outcomes. However, artificial nutrition entails its own risks and is costly. It is therefore appealing to instead maintain and support normal nutritional intake pre- and postoperatively [17–19].
Traditionally, patients were kept nil per os (NPO) the day before surgery and until full return of bowel function, which was often nearly a week thereafter [20]. The rationale behind this dogma was (1) to empty the stomach and decrease the risk of aspiration at induction of anesthesia (see Chap. 4); (2) to keep the bowel clean for surgery after a full anterograde bowel preparation; and (3) to avoid mechanical stress to a fresh anastomosis in the postoperative phase. Nasogastric tubes were widely used to decompress the digestive tract aiming to avoid distension of the anastomosis and to prevent pulmonary complications [20]. Meanwhile evidence has accumulated testing these assumptions. In fact, prophylactic nasogastric tube placement delays return of normal gastrointestinal function and increases pulmonary complications, without having a positive impact on anastomotic leak or wound complications [21, 22]. Early oral food intake has been shown to be safe and is well tolerated by 80–90 % of the patients [21, 23]. It also enhances patients’ comfort, decreases complications, and facilitates early discharge [21, 24]. It should be emphasized that early feeding is just one of multiple preventive measures that needs to be embedded within a comprehensive perioperative care pathway.
2.
Fluid management. Optimal perioperative fluid administration has been a matter of debate over the last decade (see Chap. 11), and multiple randomized trials have been conducted to compare liberal versus more restricted fluid regimens. Traditionally, surgeons and anesthetists both opted for rather liberal administration of intravenous fluids in order to prevent hypotension and hypoperfusion of organs and of the anastomosis in particular. These are potentially devastating complications, but excess administration of fluids and especially of saline also has profound pathological effects which have been summarized recently [15]. These include pulmonary edema, metabolic acidosis, and acute kidney dysfunction. Furthermore, splanchnic edema was shown by a German group to jeopardize anastomotic safety [25]. Maintaining gut perfusion both with adequate oxygen and nutrient delivery but also perfusion pressure is important to maintain gut function. Studies showing extreme fluid restriction demonstrate poor return of gut function [26], likely due to hypoperfusion, whereas “liberal” fluid regimes can lead to mucosal edema and gut dysfunction.
Lobo and coworkers clearly demonstrated in a randomized study from 2002 that fluid overload had significant effect on intestinal recovery [27]. Gastric emptying times were nearly doubled in the “liberal” group, delaying first flatus and stool by 1 and 2.5 days respectively. Compared with the restricted group, patients in the liberal group suffered from more complications and stayed 3 days longer in hospital after colonic resection. The same group summarized the available evidence in 2012 and pointed out confusion that has resulted from the terms “liberal” and “restricted.” Both inadequate hydration and fluid overload have negative impact on complications and length of stay [28]. The aim of perioperative fluid management should be to maintain normovolemia (fluid balance) while avoiding salt and water excess. They suggest that fluid administration between 1.75 and 2.5 l/day for patients without on-going losses is optimal and found significantly worse outcomes in patients gaining more than 3 kg in the postoperative period [27, 28].
Proper fluid administration is likely important in the optimal perioperative care for the prevention of ileus. Intravenous administration can be limited within enhanced recovery pathways and should aim for “zero fluid balance” with minimal weight gain only. A balanced crystalloid solution should be preferred over normal saline and can be combined in the early postoperative period with low dose vasopressors or boluses of colloids if needed [15, 28–30]. Issues remain with how to treat low blood pressure postoperatively, which may be exacerbated by the neuraxial blockade. Recent studies have shown that intraoperative and early postoperative hypotension is transient and can be counteracted by administration of low dose vasoactive agents without increased risks for renal insufficiency [31, 32]. However, the use of these agents is generally limited to patients in monitored settings.
Current recommendations for how to achieve “zero fluid balance” are somewhat vague in terms of concrete numbers but result in no or only minimal weight gain (in the region of 2 % or 1.5 kg for a 70 kg man) in the postoperative period. The value of “Goal-directed therapy” using a hemodynamic monitoring tool and a management protocol to optimize cardiac performance has not been demonstrated in non-high-risk colorectal surgery patients [29, 30, 33]. Modern perioperative pathways include a whole array of measures to maintain homeostasis and to avoid electrolyte and fluid imbalance in the perioperative phase (Fig. 12.2, Table 12.1); these include allowing clear fluids up to 2 h of surgery, no bowel preparation, carbohydrate loading, early oral intake, and early discontinuation of IV fluids.
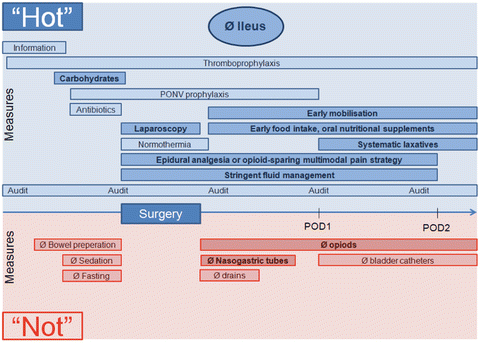

Fig. 12.2.
Enhanced recovery pathway for prevention of postoperative ileus. Modern enhanced recovery strategies bundle a multitude of preventive measures (“Hot”) in order to prevent postoperative ileus. Omission of counterproductive actions (“Not”) complements the comprehensive perioperative pathway. Best results are obtained by complete application of the protocol. The most relevant measures for the prevention of ileus are highlighted (bold, dark) and refer directly to the pathophysiological risk factors displayed in Fig. 12.1.
3.
Surgical considerations: The incidence and duration of POI appears to be commensurate with the degree of surgical trauma [2, 3, 11, 12]. It is therefore partially “in the surgeon’s hands” to diminish the surgical aggression by gentle handling of tissues and minimizing manipulation of adjacent organs and reducing blood loss. Minimally invasive surgery assumes a particularly important role. In a retrospective analysis, postoperative nasogastric decompression was required in 17.8 % of patients after open colectomy while this was necessary in only 3.7 and 4.5 % of patients after straight laparoscopic and hand-assisted resections, respectively [12]. Small bowel obstruction/ileus appeared to be the most frequent cause for readmission after ileostomy closure [7]. A recent systematic review summarizes the available evidence of 4 RCTs and 645 patients of stapled versus hand-sewn anastomosis. One of the main findings was a significantly reduced risk for small bowel obstruction in the stapled group attaining an OR of 0.54 (95 % CI 0.30–0.95).
4.
Anesthetic considerations: The anesthetist also has a role in the prevention of POI. Perioperative anesthetic drugs, analgesic techniques, and the timing and quantity of intravenous fluid therapy can affect the incidence of ileus. The use of nitrous oxide has been shown to increase the risk of postoperative nausea and vomiting (PONV) and is best avoided [34]. Oxygen-enriched air combined with a short-acting anesthetic agent such as Desflurane or Sevoflurane is standard practice although the use of Total Intravenous Anesthesia (TIVA) using target-controlled propofol infusions may reduce the incidence of postoperative nausea and vomiting. Whilst PONV itself does not directly increase the incidence of ileus, it does prevent the patient taking oral opioid-sparing analgesics which results in the administration of higher doses of parenteral opiates (which are a risk factor for POI). Early enteral feeding also promotes gut function and enables the cessation of intravenous fluids which if continued are a risk factor for ileus. PONV is a significant problem after major surgery and prophylaxis using a 5HT3 antagonist such as Ondansetron should be routinely administered (see Chap. 8). The use of a single dose of dexamethasone as an antiemetic is also useful but there is still uncertainty in its routine use in cancer surgery.
5.




Opioid-sparing analgesia: Thoracic epidural anesthesia (TEA) has traditionally been used in open surgery as the gold standard for postoperative analgesia as well as having other proven benefits of reducing the stress response (Level 1 evidence), reducing deep vein thrombosis (level 1 evidence), reducing pulmonary complications (level 1 evidence), reducing the incidence of ileus (level 1), and reducing negative nitrogen balance and fatigue (level 2 evidence). The sympathetic block achieved by TEA can improve gut motility by unopposed parasympathetic activity; however the arteriolar dilatation which also occurs can lead to hypotension and a reduction in gut perfusion so it is imperative to ensure the patient is normovolemic and maintains the blood pressure with vasopressor infusions rather than give lots of intravenous fluid which otherwise predisposes to ileus. This hypotensive side effect is minimized by using a thoracic epidural. The use of intravenous lidocaine as the main analgesic component has been shown to be both efficacious and improve the return of gut function, and adding opioid slows down gastrointestinal recovery [35, 36].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





