Peritoneal dialysis (PD) was the first successfully used modality of renal replacement therapy in acute kidney injury (AKI) patients. However, its use progressively declined after 1970 due to the greater convenience of acute hemodialysis, and it is now predominantly practiced in developing countries because of its lower cost and minimal infrastructure requirements. Recently, however, interest in using PD to manage selected AKI patients has been increasing (Ghaffari, 2013b), and a meta-analysis suggests outcomes equivalent to those with HD (Chionh, 2010).
I. INDICATIONS
A. Advantages. PD offers several advantages over hemodialysis in AKI. It is technically simple, with minimal infrastructure requirements and often lower cost. It may be the better option for the patient with difficult vascular access. Solute and water removal is gradual, with less potential for the development of disequilibrium syndrome, cardiovascular stress, and abrupt reductions in blood pressure. These potential benefits may, in turn, reduce the risk of renal and cardiac ischemia, fluid and electrolyte imbalance, and intracranial fluid shifts. No extracorporeal circulation is required, thus reducing the potential proinflammatory changes that can occur with the exposure of blood to synthetic tubing and membranes. Taken together, these factors could potentially be beneficial in permitting more rapid recovery of renal function.
Besides the classical indications (volume overload, electrolyte disorders, uremic symptoms, or acid–base disturbances), acute PD can also be used to maintain volume control in patients with congestive heart failure (CHF) functional class IV, to control hyper- or hypothermia, and to treat necrotizing pancreatitis with peritoneal lavage. Acute PD is increasingly being used in cases of advanced chronic kidney disease (CKD) presenting urgently with uremia or fluid overload, a scenario described as “urgent start PD.”
In the setting of natural disasters such as earthquakes, when several victims will develop AKI and when damage to infrastructure make access to power, clean water, and facilities for water treatment challenging, PD can be an important and life-saving renal replacement modality. Table 24.1 outlines the advantages and disadvantages of PD to treat patients with AKI.
B. Limitations. PD is relatively contraindicated in patients with recent abdominal surgery, large abdominal hernias, adynamic ileus, intra-abdominal adhesions, peritoneal fibrosis, or peritonitis. Since volume and solute removal are slow and at times unpredictable, PD is not as safe and efficient as extracorporeal blood purification techniques for the treatment of certain emergencies, such as acute pulmonary edema, life-threatening hyperkalemia, and drug overdoses. The ability of PD to achieve adequate doses in hypercatabolic AKI has been a subject of controversy. Some authors have expressed concern over PD adequacy in these situations (Phu, 2002). However, there are also reports of positive outcomes associated with PD in hypercatabolic AKI patients, especially when intensive PD regimens were used (Chitalia, 2002; Ponce, 2012b).
PD increases intra-abdominal pressure, which may lead to impaired diaphragm mobilization, decreasing pulmonary compliance and ventilation, and this may cause or worsen respiratory failure. However, patients on PD generally maintain their vital capacity and respiratory volume, and PD is seldom the cause of ventilation impairment in patients without pulmonary disease. Another possible limitation of PD in AKI is that associated protein losses may aggravate malnutrition. Protein supplementation, either enteral or parenteral (1.5 g/kg per day) has been recommended for AKI patients on PD.
Advantages and Disadvantages of Peritoneal Dialysis in Acute Kidney Injury | |
Advantages | Disadvantages |
Simple to initiate | Needs an intact peritoneal cavity with adequate peritoneal clearance capacity |
Can be initiated anywhere | Adequacy may be of concern in hypercatabolic patients |
No need for highly skilled personnel | May not be adequate for patients with severe acute pulmonary edema or life-threatening hyperkalemia |
No need for vascular access | Ultrafiltration and clearance cannot be exactly predicted |
No need for expensive equipment | Infection (peritonitis) can occur |
No exposure of blood to plastic | The standard buffer used is lactate |
No need for anticoagulation | Concern about protein losses |
Minimum blood loss | Can aggravate hyperglycemia |
Possible less negative impact on recovery of renal function | Can impair respiratory mechanics |
May be of special benefit in selected patients (children, or patients with heart failure, hemodynamic instability, bleeding diathesis) |
|
Is a form of continuous renal replacement therapy |
|
The high glucose concentrations in peritoneal dialysate may cause hyperglycemia, even in nondiabetic patients. This is easily correctable through intravenous, subcutaneous, or intraperitoneal administration of insulin. Peritonitis is a potential problem. Older studies reported a high frequency of peritonitis. However, with better catheter implantation techniques, improved connectology, and automated methods, the incidence has been reduced and the risk is similar to the incidence of infections with extracorporeal blood purification for AKI (Ponce, 2011a).
II. TECHNICAL ASPECTS
A. Peritoneal access. Safe and efficient access to the peritoneal cavity is a crucial factor for PD success. For many years, bedsides insertion of a rigid catheter using a trocar was the standard technique to access the peritoneal cavity for acute PD. This technique is still used routinely in many parts of the world, but its use has declined with the introduction of simple procedures for insertion of a flexible, cuffed Tenckhoff catheter, which provides the optimal access for PD. Depending on availability, a single- or double-cuff Tenckhoff catheter—either straight or swan neck—can be used in AKI. The advantages of a Tenckhoff catheter over the rigid catheter include having a lower incidence of leakage, larger-diameter lumen, and side holes resulting in better dialysate flow rates, and less obstruction as well as a decreased incidence of peritonitis. Furthermore, the rigid catheters need to be removed after 3–5 days, while the flexible, cuffed catheters can be left in indefinitely. Thus, if the patient does not recover renal function, the catheter may be used for chronic dialysis. Of course, it may be necessary to use alternative catheters with a rigid stylet, or even improvised options such as nasogastric tubes or surgical drains, in resource-poor environments where flexible, cuffed catheters are not available or are too costly.
Tenckhoff catheters can be inserted under local anaesthesia at the bedside, in a designated treatment room, or in a surgical theater. In a patient with previous abdominal surgery, laparoscopic or open technique is preferred, and this will usually require an operating room and a surgeon. In patients without previous surgery, no method of insertion is proven to be superior to any other. Rather, the method of implantation should be based on local availability of skills, equipment, and consumables. The bedside insertion utilizes a modified Seldinger approach with a guidewire and “peel-away” sheath and is a method practised by many nephrologists. The catheter is inserted as a blind procedure and therefore this method should be avoided, if possible, in those patients who have a midline surgical scar or history to suggest intra-abdominal adhesions. For details of catheter insertion mehods see chapter 23.
B. PD solutions. Commercially prepared PD solutions are optimal because they have the advantage of minimizing the risks of errors in mixing fluids and of contamination, and of incorporating standardized and generally accepted connectology. Where these are not available because of logistical problems or costs, locally mixed fluids can be used, but sterile production and mixing of solutions as well as use of sterile connection devices are imperative. Such locally made PD fluids can be produced from physiological intravenous fluids by adding glucose and bicarbonate.
The composition of standard PD solutions is shown in Table 22.1. Other commercial intravenous solutions that can relatively easily be converted into dialysis fluids include Ringer’s lactate, Hartmann’s solutions, half normal saline, and Plasmalyte B. Standard PD solutions generally use lactate as a buffer; this is converted to bicarbonate mainly through liver and muscle pyruvate dehydrogenase enzymes. In critically ill AKI patients (such as those with shock, poor tissue perfusion states, liver failure, etc.), there may be impaired conversion of lactate to bicarbonate, which can aggravate metabolic acidosis. In such patients, bicarbonate-containing PD solutions may be preferable. However, one small study randomized 20 AKI patients to treatment with either lactate- or bicarbonate-based PD and showed that while bicarbonate PD solution allowed better correction of metabolic acidosis and was associated with better hemodynamic stability, there were no differences in patient outcomes when compared to standard lactate solution (Thongboonkerd, 2001).
C. PD modalities. The process of dialysate instillation and removal can be automated with a PD cycler. The advantage of this system is that it can be set up by a trained staff member to reduce the risk of complications. It reduces nursing time as all cycles are automatic, and there is some suggestion that peritonitis is less likely. Automated cyclers have been used extensively to perform PD in AKI, particularly when high-volume peritoneal dialysis (HVPD) is used. However, in a resource-poor setting, cyclers may be unavailable or too expensive.
The choice of the type of PD to be utilized should be based on the experience of the medical and nursing team, available resources, the safety and efficacy of the technique, and the needs of the individual patient. An illustration of various techniques applicable to AKI is provided in Figure 24.1 and Table 24.2.
1. Intermittent PD (IPD). This is the PD technique that historically has been most frequently used in AKI and it is still the most common, being routinely practiced in many parts of the world. Patients are treated for 48–72 hours, or occasionally longer, with rapid installation and drainage of fluid and a dwell time of 30–60 minutes. A trocar-style PD catheter is traditionally used and removed after the dialysis treatment is completed, but Tenckhoff catheters are a better option and are increasingly available. Since the dialysis is interrupted when the catheter is removed, the weekly small-solute clearance is limited and might be inadequate in hypercatabolic, critically ill AKI patients. There are no recent large studies addressing this issue. Modeling suggests that IPD can deliver appropriate amounts of dialysis in a fairly broad range of clinical circumstances, depending on the degree of residual renal function (Guest, 2012).
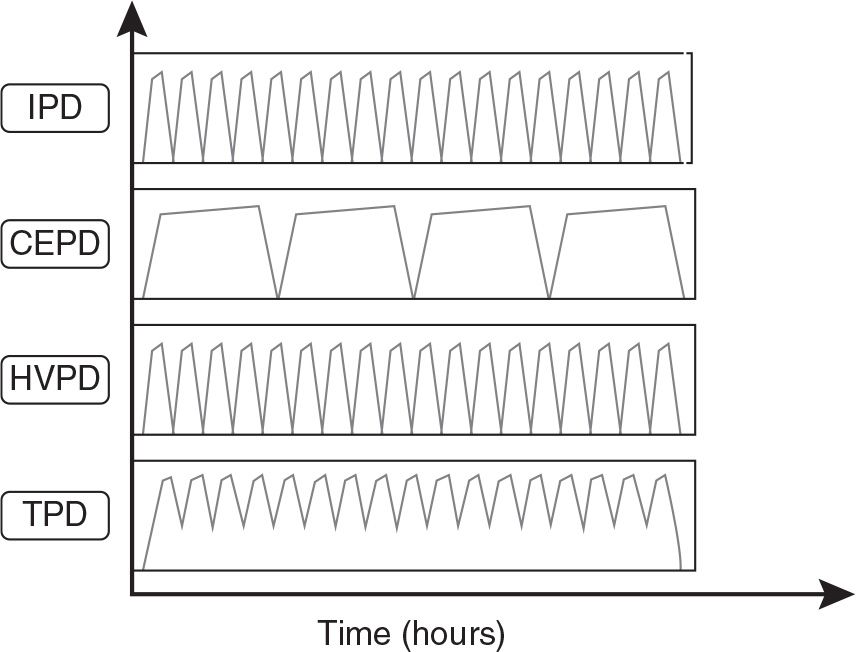
FIGURE 24.1 Illustration of the techniques of PD used for AKI patients. IPD, Intermittent PD; CEPD continuous equilibrated PD; HVPD, high volume PD; TDP, tidal PD.
Different Types of Peritoneal Dialysis and Selected Features | |
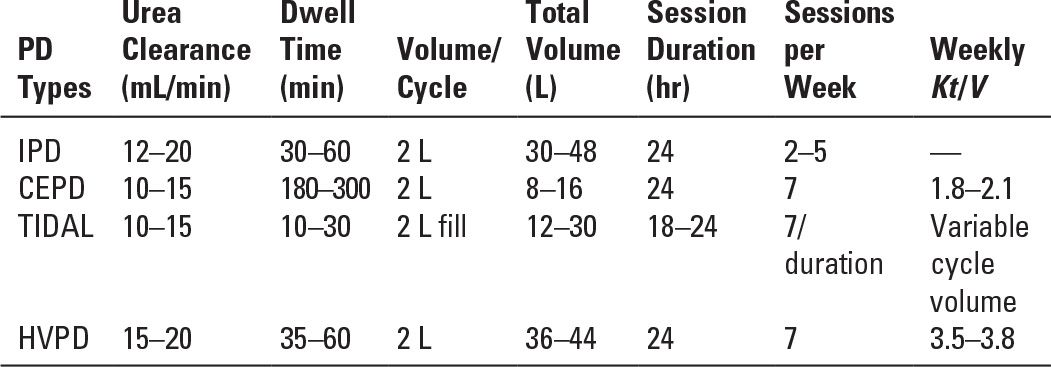
2. Continuous equilibrated PD (CEPD). This type of PD is similar to CAPD. A dwell time from 2 to 6 hours is typical, and the CEPD can be performed manually or with a cycler. Several reports of limited number of patients successfully treated by this method can be found from the 1980s onward. The clearance of small molecules and fluid removal with this methodology depend on the frequency and volume of exchanges and need to be determined based on the clinical status of the patient.
3. Tidal PD (TPD). TPD is performed with a dedicated cycler. In TPD, an initial large infusion of PD solution is followed by drainage of a portion of the dwell volume, typically 50%–75% of the initial volume, which is then replaced with fresh solution, restoring the initial intraperitoneal volume at each cycle. TPD may result in higher small-MW-solute clearances than CEPD, though not all studies have found this. TPD also may reduce the frequency of pain on drainage of dialysate from the abdomen.
4. High-volume PD (HVPD). HVPD is a continuous modality designed to achieve high small-MW-solute clearances. It requires an automated cycler and a Tenckhoff catheter. Each day the total PD solution delivered ranges from 36 to 44 L with a 30- to 50-minute dwell time. The efficacy of HVPD has been tested in several prospective studies involving seriously ill AKI patients in Brazil. With HVPD, a weekly Kt/V of 3.8 ± 0.6 could be delivered, and the mortality rate was around similar to that in AKI patients treated with intermittent or extended daily hemodialysis (Gabriel, 2008).
III. PRESCRIBING AND DOSING OF ACUTE PD. The most appropriate prescription and dose for PD in the management of patients with AKI is poorly defined because there are only a limited number of trials available to compare treatment modalities, the studies that have been done have methodological flaws, and the dose of dialysis used has varied widely (Chionh, 2010).
Where resources permit and where a cuffed catheter can be placed, using HVPD targeting a Kt/V urea of 0.5 daily (3.5 weekly) is associated with outcomes comparable to that of daily HD; targeting higher doses does not appear to improve outcome (Gabriel, 2008). A review of the literature suggests that such a high dose may not be necessary for many patients with AKI and that targeting a daily Kt/V of 0.3 (2.1 weekly) with a modified CEPD approach may be adequate for many patients (Cionh, 2010; Ivarsen, 2013). This may be particularly helpful in developing countries where resources are limited, costs are critical, and AKI is more often caused by infections, volume contraction, obstetrical problem, and so on, rather than complex postsurgical complications with multiorgan failure.
During the initial 24 hours of therapy, the cycler dwell time needs to be dictated by the clinical circumstances of the patient. Short cycle times (every 1–2 hours) with dwell volumes of 1.5 or 2 L may be necessary to correct hyperkalemia, fluid overload, or metabolic acidosis. Thereafter, the cycle time can be increased but generally not beyond 4–6 hours. Ultrafiltration is regulated by adjusting the dextrose concentration of the solution and by shortening the cycler dwell time.
A. How to prescribe acute PD. As the dialysis requirements of a patient may change from day to day, it is prudent to write PD orders for only 24 hours at a time, reassessing and altering the prescription as indicated. A standardized form for acute PD prescriptions is helpful in assuring that the specifications of the procedure are complete and clear for the nursing staff responsible for its delivery (Table 24.3).
1. Exchange volume. Choice of exchange volume is dictated primarily by the size of the peritoneal cavity. An average-sized adult can usually tolerate 2-L exchanges, but in smaller patients, those with pulmonary disease, and those with abdominal wall or inguinal hernias, the volume should be reduced. Although initiating acute PD with a 2-L exchange volume is standard, some nephrologists prefer to start with smaller volumes (1–1.5 L) for the first few exchanges to minimize the risk of leaks. Otherwise, one should not reduce the exchange volume without good reason as this leads to lower clearances. In large or very catabolic patients, an exchange volume of 2.5–3 L may be helpful to augment the efficiency of dialysis.
Acute Peritoneal Dialysis Sample Orders | |
A. Nursing orders
1. Dialysis to run________hours
2. Exchange volume: ________L
3. Warm dialysis fluid to 37°C
4. Exchange time: Inflow 10 minutes
Dwell________minutes
Outflow 20 minutes or as long as fluid drains freely
DO NOT LEAVE FLUID IN ABDOMEN
5. Strict intake and output to be kept on fluid intake–output record
6. Dialysate balance to be recorded on peritoneal dialysis record
7. Dialysis fluid running balance to be maintained at: ________L
8. Dialysate solution: ________%
9. Additives to dialysate:
Medication dose frequency
________ ________/2 L q exchange or ×________exchanges
________ ________/2 L q exchange or ×________exchanges
10. Heparin: 1,000 units/2 L q exchange: yes/no
11. Turn and position patient p.r.n. for optimum outflow
12. Vital signs q________hours
13. Catheter care and dressing change every day
14. Withdraw 15 mL dialysis fluid from catheter port every morning during dialysis and send for cell count with differential, and culture and sensitivity: yes/no
B. Blood draw orders:
1. BUN, creatinine, HCO3, Na, K, Cl, and glucose 8 a.m. and 6 p.m. each day during dialysis
C. Notify physician immediately for:
1. Poor dialysate flow
2. Severe abdominal pain or distention
3. Bright red blood or cloudy dialysate drain
4. Dialysate leak or purulent drainage around catheter exit site
5. Blood pressure of <________ mm Hg systolic
6. Respiration rate of >________/minute, or severe shortness of breath
7. Temperature of >________°C
8. Two consecutive positive exchanges
9. Single positive exchange balance (dialysate-IN – dialysate-OUT) of ≥1,000 mL
10. If negative balance exceeds ________ L over ________ hours
BUN, blood urea nitrogen.
2. Exchange time. This is the combined time required for inflow, dwell, and drain. If the aim is to maximize small-solute clearance, the exchange time should be relatively short at about 1–2 hours, but in CEPD, longer times are routine.
a. Inflow time. Inflow is by gravity or hydraulically pumped with a cycler and usually requires about 5–10 minutes (200–300 mL/min). Inflow time is dictated by the volume to be infused and, with manual systems, the height of the dialysis solution above the patient’s abdomen. It may be prolonged due to kinking of the tubing or increased inflow resistance by intra-abdominal tissues in close proximity to the catheter tip. On initiation of acute PD, some patients may experience pain or cramping with inflow of PD solution. This may result from the hypertonic and acidic nature of the PD fluid and often improve with time but, when severe, may be relieved by slowing the dialysate inflow rate for several exchanges. Otherwise, inflow time should be kept to a minimum to maximize dialysis efficiency. Cold PD solution can result in discomfort and hypothermia, and so the solution should be warmed to 37°C before infusion.
b. Dwell time. The dwell period is the time during which the total exchange volume is present in the peritoneal cavity (i.e., the time from the end of inflow to the beginning of outflow). When initiating PD in acutely ill and catabolic patients, the usual dwell time is 30 minutes to achieve an exchange time of 60 minutes. With a 2-L exchange volume, as much as 48 L of fluid can be exchanged daily. Given a peritoneal membrane with average transport characteristics, the urea concentration in the drained dialysate will be approximately 50%–60% of that in the plasma (D/P ratio of 0.5–0.6 at 1 hour). Thus, with an aggressive dialysis exchange rate of 2 L/hr, the plasma urea clearance could approximate 24–29 L per day (0.5–0.6 × 48 L per day) or 168–202 L per week. If the patient is not very catabolic, a longer dwell time (e.g., 1.5–6 hours) can often be used. With a 4-hour exchange time (dwell time 3.5 hours), the dialysate urea concentration is, on average, 90% of that in the plasma (D/P ratio of 0.9 at 4 hours). This leads to a plasma urea clearance of at least 11 L per day (0.9 × 12 L per day), or 77 L per week. Assuming an ultrafiltration rate of 1 L per day, this would add 6.3 L of clearance per week, making a total clearance of 83 L per week. In terms of weekly Kt/V urea (see what follows), the weekly clearance of 83 L is the (K × t) term. For a 70-kg male patient with a V of 42 L, weekly (K × t)/V would be 83/42 or about 2.0.
c. Outflow time. Outflow of spent dialysate is by gravity and usually requires 20–30 minutes. Outflow time depends on the total volume to be drained, the resistance to outflow, and, with manual methods, the difference in height between the patient’s abdomen and the drainage bag. In many patients, particularly those with large abdomens, the first exchange may not drain completely (often only 1–1.5 L is retrieved) due to initial filling of poorly draining areas of the abdomen. As long as marked abdominal distension is not present, a second exchange of 2 L can be cautiously instilled. Subsequent drainage usually proceeds normally.
3. Choosing the dialysis solution dextrose concentration
a. Standard 1.5% dextrose (glucose monohydrate). This concentration of dextrose (approximately 1,360 mg glucose/dL [75 mmol/L]) will, in general, exert an osmotic force sufficient to remove 50–150 mL fluid/hr (although this may vary from patient to patient) when using a 2-L exchange volume and a 60-minute exchange time. This ultrafiltration rate could translate into fluid removal of 1.2–3.6 L per day.
b. Higher concentrations of dextrose. Greater fluid removal can be achieved with higher dextrose concentrations. A 4.25% dextrose solution can result in an ultrafiltration rate of 300–400 mL/hr. Acutely, this degree of fluid removal can be required for the treatment of congestive heart failure or marked volume overload. However, continued use of the 4.25% solution could theoretically result in the removal of 7.2–9.6 L per day and cause marked hypernatremia. In practice, this degree of fluid removal is rarely required. Available dextrose solutions (i.e., 1.5%, 2.5%, or 4.25% exchanges) can be adjusted to provide the level of ultrafiltration desired. Once the patient is euvolemic, one can resume using 1.5% solution for all exchanges.
4. Dialysis solution additives. When injecting any additive into PD solution bags, sterile technique must be followed in order to prevent bacterial contamination of the dialysis solution and peritonitis.
a. Potassium. Standard PD solutions contain no potassium (K). In general, after the initial exchanges, serum K concentrations are within the normal range, unless the patient is very catabolic. In fact, losses of K can be high in acute PD. Such removal may cause serious K depletion and cardiovascular instability. This can be prevented or corrected by adding K to the dialysis solution. When serum K is lower than 4 mM, K 4.0–5 mM can be added to the PD solutions to minimize the risk of hypokalemia.
b. Heparin. Sluggish dialysate flow from catheter obstruction by fibrin or blood clots may occur in acute PD, often as a result of the slight bleeding that may accompany catheter insertion or irritation of the peritoneum by the catheter. Heparin (500–1,000 units/L) added to the dialysis solution can be helpful in preventing or treating this problem. Because heparin is absorbed minimally through the peritoneum, there is no increased risk of bleeding.
c. Insulin. Because glucose is absorbed from the dialysis solution, supplemental insulin administration may be required for the diabetic patient undergoing acute PD. Insulin can be given subcutaneously or intravenously, or regular insulin may be added to the PD solution before infusion. The blood glucose level must be monitored closely, and the dose of insulin tailored to the needs of the patient.
d. Antibiotics. Intraperitoneal administration of antibiotics is an efficient route for treating peritonitis. In general, antibiotics should not be given intraperitoneally to treat systemic infections.
B. How to measure dose in acute PD. It is important to ensure that acute PD is delivering an adequate amount of dialysis for the AKI patient. Adequacy is generally assessed by measuring the Kt/V urea nitrogen delivered by the PD. This is done by measuring the urea concentration in representative samples of dialysate and plasma in order to calculate a D/P ratio for urea. This is multiplied by the total daily dialysate drain volume and divided by the estimated volume of distribution of urea using anthropometric equations for total body water such as the Watson equation (see Chapter 25). However, patients with AKI often are fluid loaded, and urea distribution volume often will be considerably higher than predicted by such equations.
MEASUREMENT OF DELIVERED KT/V

This is multiplied by 7 to yield the weekly Kt/V urea
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





