Fig. 5.1
Anatomy of the anal canal and rectum
Perianal space is a potential space in the area of anal verge, where a low intersphincteric tract drains. The intersphincteric space lies between two sphincters, and inferiorly it is in continuity with the perianal space. The supra levator space is an upward continuation of the intersphincteric space and is bounded superiorly by peritoneum, inferiorly by levator ani, medially by rectal wall and laterally by pelvic wall. Ischiorectal space extends from levator ani above to perineum below. Deep postanal space lies between tip of the coccyx and rectal wall between levator ani above and anococcygeal ligament below.
5.3 Epidemiology and Etiology
Anal sepsis can occur at any age though it is more common in third or fourth decade of life. In children, it is more common in babies under 12 months of age. Males are affected more frequently than females, with a male-to-female ratio of 2–3:1 (Isbister 1987). This male predominance is particularly marked in infancy. Approximately 30 % of patients with anorectal abscesses have a previous history of similar abscesses (Ramunjam et al. 1984).
Many factors have been associated with development of anal sepsis like malignancy, trauma, tuberculosis, foreign bodies, inflammatory bowel disease, simple skin infection, and actinomycosis. However, most anal sepsis originates from an infected anal gland. Obstruction of these glands leads to stasis, bacterial overgrowth, and ultimately abscess formation. This represents cryptoglandular theory of anal sepsis which is responsible for almost 90 % of all anal fistulas. Mixed aerobic and anaerobic bacteria account for 72 % of cases, while pure aerobic and anaerobic infection only accounted for 9 % and 19 % of cases, respectively (Brook and Fraizer 1997). Common organisms implicated include E.coli, bacteroides, and enterococcus species. The presence of skin-derived bacteria such as Staphylococcus indicates an abscess resulting from the secondary infection of blocked apocrine glands (Grace et al. 1982; Henrichsen and Christiansen 1986). Review of the literature shows that the incidence of gut-derived and skin-derived organisms in patients with anorectal abscesses with fistula ranged from 85 to 100 % and 0 to 38 %, respectively (Takayuki et al. 2008). The likelihood of patients developing anal fistula after drainage of an anorectal abscess is similarly higher in patients whose infection is caused by a gut organism.
5.4 Classification
5.4.1 Anorectal Abscess
Anorectal abscesses are classified according to the location of the abscess cavity. In order of decreasing frequency they are perianal, ischiorectal, intersphincteric (also called submucosal), and supralevator (Nelson 2002). One or more spaces can be involved by a given suppurative process at a time. For example, an intersphincteric abscess can extend to involve the supralevator space. From intersphincteric, supralevator, or ischiorectal space, pus can extend circumferentially to the opposite side via the deep postanal space resulting in what is called a horseshoe abscess. These abscesses can also extend to the scrotum and may cause Fournier’s gangrene; extend to the labia and can get diagnosed as Bartholin’s abscess; can extend to the thigh, calf, and abdominal wall; and lead to necrotizing fasciitis (Fig. 5.2).


Fig. 5.2
Perianal spaces
5.4.2 Anal Fistula
Many different classifications were given for fistula-in-ano; however, the most useful classification is the one described by Parks et al. in 1976 (Fig. 5.3). Parks classified fistula-in-ano on the basis of course taken by main tract in relation to the anal sphincters, i.e., intersphincteric, transsphincteric, suprasphincteric, and extrasphincteric. These groups can be further subclassified on the basis of secondary tracts and other finer anatomical details (Parks et al. 1976). The Park’s classification is not complete and in fact may not be accurate in some instances, e.g., the suprasphincteric classification as described by Park’s does not represent the natural spread of the disease. All supralevator extensions are actually upward extensions of an intersphincteric, tract, and the only other way the supralevator space can be reached is via an iatrogenic route.


Fig. 5.3
Classification of fistula-in-ano. (a) Low intersphincteric fistula-in-ano causing a perianal abscess. (b) Transsphincteric fistula causing an ischiorectal abscess. (c) High intersphincteric fistula causing a supralevator abscess. (d) Extrasphincteric fistula
Fistulas can also be classified as simple or complex. Complex fistula includes recurrent fistula, fistula with multiple tracts, anterior fistula in females, high fistulas, and fistula with extension to adjacent organs (Fig. 5.4a–c).


Fig. 5.4
(a) Recurrent fistula showing internal and external openings. (b, c) Water-can perineum with recurrent complex fistulas
5.5 Diagnosis
5.5.1 Anorectal Abscess
Patients with anorectal abscess can present in two ways, either as a draining sepsis or as contained infection. Patients with contained infection present with indolent onset of increasing pain around the anus, rectum, or buttocks. Later on, there can be severe, non-relieving, constant, throbbing pain with pressure sensation. Pain is commonly associated with systemic symptoms like fever and sweating. Patients with draining anorectal abscess also present with complaints of offensive discharge on their undergarments or on toilet paper, along with the history described above. Onset of discharge is associated with relief of pain.
On examination, findings vary according to anatomic location of anorectal abscess. Examination under anesthesia may be required in some cases. Perianal and ischiorectal abscess will have erythema, swelling, tenderness, and fluctuation over their respective sites. Intersphincteric or submucous abscess may not have any visible external manifestation despite patient’s complaint of severe excruciating anal pain. Digital rectal examination may not be possible because of severe tenderness. However, digital examination, if possible, will demonstrate tender fluctuant mass in these cases. Evaluation under anesthesia or MRI maybe required in cases where digital rectal examination is not possible or the diagnosis is in doubt. Supralevator abscess represents upward extension of intersphincteric abscess.
5.5.2 Anal Fistulas
A fistula-in-ano represents the chronic phase of ongoing anorectal sepsis. A previous history of anorectal abscess drained either spontaneously or surgically can usually be elicited. Patients often report a cyclical pattern of pain, swelling, and drainage. Moisture can cause skin irritation, excoriation, and pruritus. Crohn’s disease should be excluded in patients with history of chronic diarrhea or abdominal pain.
Physical examination usually identifies one or more external openings with or without granulation tissue and a surrounding scar. Occasionally, the external opening may be subtle and appreciated only after closer inspection of an indurated area. Uncommonly, the patient may present during the phase where the external opening has temporarily closed. Palpation may elicit tenderness, expression of pus, and a fibrotic cord extending toward the anus. The external tracts are usually not felt during its entire course, unless it is a very low fistula. Several external openings may be present because of branching complex fistulous tracts, a condition called as watering-can perineum. It is generally not recommended to do probing of the fistula track in the office setting, but digital anorectal examination, anoscopy, and proctoscopy may help discover the internal opening (Fig. 5.4b) and the presence of any other rectal disease. The internal opening is often seen at the dentate line which is consistent with cryptoglandular theory of anorectal sepsis. An enlarged papilla is often present at the site of the internal opening. Most common site for internal opening is posterior midline as most anal glands are located posteriorly. The intersphincteric tract may be felt as a submucosal induration on digital rectal examination.
In 1900, Goodsall described a simple rule that uses the location of the external fistula opening to predict the location of the internal opening. Fistulas with external openings anterior to the anterior half of the anus, usually track in a radial fashion directly into the anal canal (Fig. 5.5). Fistulas with an external opening in the posterior half of the anus usually track in a curvilinear fashion to originate from the posterior midline. Exception to this rule includes multiple external openings, and external opening which is present anteriorly, more than 3 cm. from the anal verge. In most of these cases, the internal opening will be in the midline posteriorly.


Fig. 5.5
Goodsall’s rule
Ciroco and Reilly found that Goodsall’s rule was inaccurate in patients with anterior external opening because almost 71 % of these fistulas have internal opening in midline anteriorly (Cirocco 1992). The Goodsall’s rule also fails in cases of fistula with associated carcinoma or Crohn’s disease (Fazio 1987). The Goodsall’s rule is not accurate in a fair number of patients and cannot be relied upon in all the cases.
5.5.3 Special Studies
5.5.3.1 Sigmoidoscopy and Colonoscopy
Sigmoidoscopy should be performed in all patients with symptoms suggestive of anal fistula in order to rule out other pathologies like proctitis or neoplasia. Presence of symptoms suggestive of inflammatory bowel disease or neoplasia necessitates need for full colonoscopy.
5.5.3.2 Fistulography
Contrast material-enhanced fistulography was the first imaging modality used. In fistulography, the external opening is catheterized with a fine cannula, and a water-soluble contrast agent is injected gently to define the fistula tract (Fig. 5.6). Fistulography was the most widely used imaging modality. However, it was of limited sensitivity due to its poor localization of the internal opening, absence of precise anatomic landmarks, and lack of direct demonstration of the sphincter complex and levator ani sling. There was a high probability of missing the secondary fistulous tracks, due to frequent non-filling of the side branches with subsequent high rates of postoperative recurrence (SteveHalligan and JaapStoker 2006; Jones Jennifer and Tremaine William 2005; Kuijper and Schulpen 1985). The current use of fistulography may be limited to finding out the presence or absence of fistula, in patients with external opening situated at a distant site, or in a nonhealing postoperative wound. The dye entering the anal canal will confirm the presence of a fistula. The presence of a long tract or a tract coursing toward the anal canal without entering it may be suggestive of a fistulous tract. Even for these cases, there are better imaging modalities available.


Fig. 5.6
(a) Dye injected through distal opening on the thigh. (b) Fistulogram through the external opening showing communication with anal canal
5.5.3.3 Endoanal Ultrasonography
Anal endosonography developed by Clivev Bartram was the first technique to directly depict the anal sphincter complex in detail (Law and Bartram 1989). It is simple, rapid, and well tolerated by patients. Because of its ability to demonstrate the presence and extent of anal sphincter disruption, the technique has attracted considerable attention (Sultan et al. 1993) (Fig. 5.7). The internal sphincter is visualized as a hypoechoic ring encircling the anal canal, whereas the external sphincter is of mixed echogenicity. The intersphincteric space and longitudinal muscle lie between these and are of mixed echogenicity and are easily identified by using modern 10-MHz transducers (Frudinger et al. 2002).
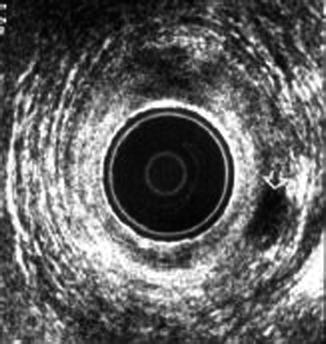

Fig. 5.7
Endoanal USG showing an intersphincteric abscess
Internal opening of the fistulous tract and intersphincteric fistulas is usually very well visualized at anal endosonography. Transsphincteric fistulas are revealed by tracts that cross the external sphincter to reach the ischioanal fossa. Extensions from primary tract are revealed as hypoechoic fluid collections. The gas reflections from hydrogen peroxide highlight the track and may help to locate the site of the internal opening. However, insufficient penetration of the ultrasound beam beyond the external sphincter, especially with high-frequency transducers, limits the ability to image ischioanal and supralevator infections, with the result that extensions from the primary tract may be missed at endosonography. In patients with recurrent disease, anal endosonography may not be reliable, because of its inability to distinguish infection from fibrosis (Choen et al. 1991). Three-dimensional ultrasonography may be more useful in detecting smaller lesions (Fig. 5.8).


Fig. 5.8
Three-dimensional endoanal USG
5.5.3.4 Computed Tomography (CT) Scan
Computed tomography with rectal and intravenous contrast had been used for evaluation of fistula-in-ano; however, it did not gain popularity because of poor visualization of levators and sphincter complex. At present, CT scan is indicated in patients with suspected supralevator abscess or high-complex fistula to rule out associated pelvic pathology (Fig. 5.9).


Fig. 5.9
CT scan. (a, b) Reconstructed CT scan showing a supralevator tract
5.5.3.5 Magnetic Resonance Imaging (MRI)
MR imaging is the most accurate method for determining the presence and course of anal fistula (Lunniss 1992). The success of MR imaging for preoperative classification of fistula-in-ano is a direct result of the sensitivity of MR for tracts and abscesses combined with high anatomic precision and ability to image in surgically relevant planes (Fig. 5.10). Endoanal coil provides excellent anatomic detail of the anal sphincters. However, because of its limited field of view and limited accuracy in complex supralevator types, it has not become very popular. The body coil or the pelvic surface coil is more frequently used for imaging the pelvis in fistula patients (SteveHalligan and JaapStoker et al. 2006). Spencer and colleagues independently classified 37 patients into those with simple or those with complex fistulas on the basis of MR imaging and EUA and found that MR results were better predictors of outcome, with positive and negative predictive values, respectively, of 73 and 87 % for MR and 57 and 64 % for EUA (Spencer et al. 1998). In a study by Beeets-Tan et al., preoperative MRI had a sensitivity and specificity of 100 % and 86 %, respectively, for identification of fistulous tract and sensitivity and specificity of 96 % and 90 % for preoperative detection of internal opening (Beets Tan et al. 2001). Intravenous contrast studies are more specific and can attain similar results of local MRI fistulogram. So, it should be routinely included in MRI protocols of anal fistula examination, even with no abscess or collection seen at the precontrast images. Color Doppler can help to check patency of the fistulous track and can be added as complementary noninvasive investigation.


Fig. 5.10
Endocoil MRI showing an intersphincteric abscess
5.5.3.6 Anorectal Manometry
The role of anorectal manometry to diagnose fistula is very limited. It may be more useful in managing fistulas with preoperative incontinence or coexisting obstructed defecation syndrome. It may also prove to be useful in cases of medical litigation if the anal pressures are documented before and after surgery.
5.5.3.7 Fistuloscopy
Anorectal fistuloscopy using flexible uretroscope is a new modality under evolution that is useful intraoperatively to indentify primary fistulous opening, multiple, complex tracts or iatrogenic tracts (Johnson et al. 2005). It can be a promising tool in diagnosis and management of complex fistula in times to come.
5.6 Treatment
5.6.1 Anorectal Abscess
Perianal abscess is a surgical emergency and should be treated by urgent incision and drainage (Whiteford 2005). Depending on the condition of the patient and location of the abscess, drainage can be done either as an outpatient procedure or may be done under anesthesia in an operating room. Conditions such as cellulitis without fluctuation, failed drainage in the office, abscesses with symptoms and signs of sepsis, or extensive abscesses with supralevator extension are more appropriately treated in the operating room, where a thorough examination under anesthesia can ensure optimal diagnostic evaluation and drainage. Although incision and drainage is effective in resolving the acute abscess, the patient is at risk for developing chronic anal fistula or recurrent perianal sepsis or both. These conditions occur in 35–50 % of patients after a first-time perianal abscess (Hamadani et al. 2009). Gender, smoking history, perioperative administration of antibiotics, and HIV status are not risk factors for fistula formation or recurrence of perianal sepsis (Hamadani et al. 2009). The drainage should be done as close to the anus as possible in order to shorten the length of any subsequent fistulous tract. Mechanical disruption of loculi around abscess cavity may cause injury to sphincter complex or the pudendal nerve and hence should be done carefully in order to ensure adequate drainage of all pus. Routine addition of antibiotics has not been shown to improve healing time or reduce recurrence rate (Llera and Levy 1985; Stewart et al. 1985; Macfie and Harvey 1977). Antibiotics should be considered for patients with high-risk conditions such as diabetes, immunosuppression, extensive cellulitis, prosthetic devices, and valvular heart diseases (Whiteford 2005; Dajani et al. 1997). After successful drainage, pain relief is usually immediate. Postoperatively, patients are advised to use sitz bath, laxatives, and analgesics. Bleeding and drainage usually subside within a few days. The wound heals over a matter of few weeks. Follow-up is encouraged because acute abscesses occur in 10 % and of chronic fistula-in-ano occurs in up to 50 % of patients (Vasilevsky and Gordon 1984).
The patient is placed in the prone jackknife, left lateral, or in lithotomy position, and the area surrounding the abscess cavity is adequately painted and draped. Patient may be given general anesthesia or perianal field block as per location of abscess. A stab incision is made with an 11 or 15 number blade at the point of maximum fluctuation in closest proximity to the anal verge. Larger cavities may require digital or hemostat-assisted exploration to break up any undrained abscesses. In addition to adequate drainage, one should endeavor to prevent acute recurrence of an abscess by either excising the overlying skin or by inserting drainage catheter. Either of these techniques will allow any undrained pus to be expelled and decrease the chance of acute recurrences (Isbister 1987; Read and Abcarian 1979). The patient is advised pain medications and a follow-up appointment in 1–2 weeks at which point the drainage catheter is removed. Antibiotics are prescribed if indicated.
Intersphincteric, supralevator, and deep postanal abscesses are best treated in the operating room where a thorough exam can be performed. For intersphincteric abscesses, the internal sphincter is divided from its lower end to the dentate line and hemostasis is achieved. If a supralevator abscess is a result of upward extension of an intersphincteric abscess, the abscess should be drained directly into the rectum (Fig. 5.11). However, if it is a result of an ischioanal abscess, it should be drained via the ischioanal fossa (Figs. 5.12 and 5.13). Supralevator abscesses that are caused from extrapelvic diseases such as Crohn’s, diverticulitis, or appendicular abscesses may be initially drained via the rectum or the ischioanal fossa. However, pathology of the offending organ must be addressed (Gordon and Nivatvongs 1999).




Fig. 5.11
Drainage of intersphincteric abscess through the internal opening

Fig. 5.12
Diagrammatic representation of drainage of intersphincteric abscess through internal opening

Fig. 5.13
Route of drainage of ischiorectal and perianal abscess
5.6.2 Horseshoe Abscess
The horseshoe abscess is caused by an infected anal gland located in the posterior midline of the anal canal. The presence of the dense overlying anococcygeal ligament prevents the direct downward expression of an abscess. As a result, the suppuration follows the path of least resistance laterally into ischiorectal fossa, hence the term “horseshoe” (Fig. 5.14). Treatment requires unroofing of the abscess cavity through the overlying anococcygeal ligament along with counter drainage of the lateral extensions. Placement of a draining (loose) seton may prevent premature skin closure, avoid an acute abscess recurrence by providing a route of egress for the infection, and facilitate fibrosis of the fistula tract.


Fig. 5.14
Horseshoe abscess
5.6.3 Abscess and Primary Fistulotomy
Fistulotomy performed at the same sitting as incision and drainage of a perirectal abscess is termed a “primary” or “synchronous” fistulotomy. Doing a primary fistula surgery along with drainage of the anorectal abscess has been a matter of much debate. The fear of causing incontinence and the fact that more than half of all drained abscesses may develop a fistula have resulted in condemning a primary surgery for an anorectal abscess. However, a recent systematic review comparing outcome after primary fistula surgery done along with drainage of perianal abscess, compared with drainage alone, showed that fistula surgery with abscess drainage significantly reduced recurrence or persistence of abscess/fistula or the need for repeat surgery (Malik et al. 2010). Statistically, there was no significant evidence of incontinence following fistula surgery with abscess drainage. This intervention may be recommended in carefully selected patients. Another meta-analysis comparing the two procedures concluded that there was no conclusive evidence if simple drainage or sphincter-cutting procedure is better in the treatment of anorectal abscess–fistula (Quah et al. 2006). A long-term follow-up seems not to influence the results of fistulotomy group and confirms that fistulotomy is an efficient and safe treatment of anal abscess with good long-term results (Benjelloum et al. 2013). An exception is a high fistula, where fistulotomy may be associated with a risk of recurrence and incontinence.
5.6.4 Fistula-in-Ano
Surgery is the preferred modality of treatment for patients with in ano. With delay in treatment, a simple fistula can progress to chronic abscess or to a complex fistula and hence the need for timely intervention. There has been no consensus on surgical options for treating it. The existing options have not yielded satisfying results, hence the need to discover new options. Recurrence and incontinence are the two major paradoxical factors which a surgeon fears and drives him/her to tilt on one side or the other. Surgery for fistula can be performed under general, regional, or local anesthesia with sedation. Patient is placed either in lithotomy or prone jackknife position, depending on patient characteristic, location and extent of fistula, and the surgeon’s preference.
5.6.4.1 Advancement Flap
Advancement flaps have been used to close the opening in fistula since long. The success with advancement flap as a stand-alone procedure ranges from 59–72 % (Mizrahi et al. 2002; Sonoda et al. 2002). Crohn’s fistulas have a lower success rate. Repeating this procedure multiple times can further increase the success rate to as much as 90 % (Mitalas et al. 2007; Jarrar and Church 2011). Continence can deteriorate in 9–14 % of patients after this procedure. Advancement flaps consist of mucosa, submucosa, and part of the internal sphincter. The flap is lifted, edge of the flap containing internal opening. The underlying fistulous tract is excised up to the level of the internal sphincter. Here, the tract is transfixed. The flap is then advanced and sutured to close the internal defect. The outer part of the track can be curetted. In the last couple of years, there have been many articles comparing treatment with advancement flaps and anal fistula plug. A meta-analysis comparing the two methods has revealed equivalent success rates, but overall results were in favor of the fistula plug. Later, it was associated with decreased risk of incontinence, less postoperative pain, faster healing, and a superior quality of life (Fig. 5.15) (Leng and Jin 2012).


Fig. 5.15
Advancement flap
5.6.4.2 Fibrin Glue
Fibrin glue has been used as a sphincter-sparing approach for the treatment of anal fistula for 2 decades. The mixture of fibrinogen and thrombin is injected into the fistulous tract after it has been curetted out thoroughly (Figs. 5.16 and 5.17). It activates fibrinogen-forming fibrin clot. The resulting coagulum plugs the fistulous tract completely. Migration and activation of fibroblasts form a collagen network. Glue is injected through a catheter part, and patient is immobilized under general anesthesia for about 15 mts for fixation.










