Fig. 20.1
Cardiac box
Given the orientation of the heart in situ and relative size of the chambers, the right ventricle is at highest risk to an anterior penetrating injury, while the left atrium is at lowest risk. Analysis of injury patterns supports this bias. In a combined review of 3,400 penetrating cardiac wounds rates of injury were as follows: right ventricular injury 43 %, left ventricular injury 34 %, right atrial injury 18 %, and left atrial injury 5 %. Multiple chamber injury occurred in 18 % of the cases and coronary arterial injury in less than 5 % [18].
Penetrating cardiac wounds present unique physiologic challenges. Cardiac tamponade especially provides distinctive physiologic derangement. The pericardium, a fibrous and inelastic sac, lacks compliance to respond to acute bleeding. Thus, intrapericardial pressure climbs rapidly, resulting in failure of venous inflow to the heart. Decreased right and left ventricular stroke volume stimulates the adrenergic response leading to tachycardia and increased cardiac contractility. As intrapericardial pressure rises, end-diastolic pressure must rise to prevent cardiac chamber collapse and loss of filling. As intracardiac and intrapleural pressures equalize, cardiac arrest ensues.
While less common, there are a variety of additional injuries possible from these wounds that may lead to significant morbidity and mortality. Injuries to coronary arteries, valves, papillary muscles, or myocardial tissue damage may lead to cardiac dysfunction or arrhythmias. Therefore, evaluation of cardiac chambers, valves, and septae is mandatory in the perioperative or intraoperative phase of care. Transthoracic or transesophageal echocardiography is best for this purpose. Foreign body embolism and intracardiac shunts due to septal injuries may lead to major complications or death. Late complications may also complicate management, including endocarditis, suppurative pericarditis, false ventricular aneurysms, and coronary-cameral fistulae (fistula between the coronary artery and a cardiac chamber).
Radiologic diagnostic options include chest radiograph, ultrasound, echocardiogram, multidetector CT, and MRI. Chest radiography alone cannot rule out cardiac injury, although it may identify pneumothoraces, pneumopericardium, hemothoraces, retained foreign bodies, or mediastinal hematoma. Of note, the cardiac silhouette will likely not be enlarged following acute cardiac trauma so plain radiography cannot rule out injury. Ultrasound may be rapidly employed, is minimally invasive, readily available in the trauma bay, and is widely used to diagnose traumatic hemopericardium. The FAST (focused abdominal sonogram for trauma) provides excellent positive and negative predictive values despite being somewhat operator dependent [19] (Fig. 20.2). Echocardiography may assess valvular dysfunction, septal injury, wall motion, and cardiac tamponade/effusion. More sensitive than the FAST, echocardiography may detect as little as 25 mL. Unfortunately, the sensitivity of TTE may be significantly limited by body habitus, tubes, and dressings. TEE has improved sensitivity but is invasive, requires specially trained operators and sedation, and can be technically complicated by associated cervical spine, esophageal, or facial trauma. In acute penetrating cardiac trauma TEE may be most useful intraoperatively but should not delay operative intervention as indicated.
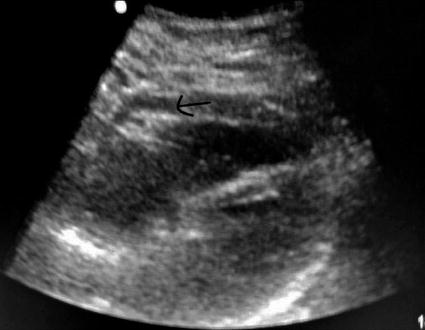

Fig. 20.2
Positive subxyphoid pericardial window on FAST, arrow indicates pericardial fluid
Both multidetector CT and MRI generally require placing patients at risk for rapid decompensation in areas with limited ability to closely monitor the patient and poor resources to acutely intervene should the patient’s clinical status deteriorate. Patients with any evidence of hemodynamic instability, signs of pericardial fluid on FAST, undrained or rapidly draining hemothorax, or indicators of shock should not travel to the radiologic suite. While blunt cardiac injury may lead to injury patterns detectable on CT/MRI, in “stable” penetrating trauma patients, these imaging modalities may be most useful to rule out other intrathoracic occult injuries (contained great vessel injury/pseudoaneurysm, tracheal, esophageal injury, etc.) rather than to assess for penetrating cardiac trauma.
Historically, patients presenting with presumed penetrating cardiac injuries, not in extremis, underwent subxyphoid pericardial window (SPW) as a diagnostic procedure to evaluate for pericardial blood. SPW involves anesthesia, surgical division of the linea alba, detachment of the xiphisternal attachments, and sharp division of the pericardium. Blood in the pericardium classically mandated further operative exploration. The use of bedside ultrasound in the trauma bay has largely supplanted SPW as the gold standard for ascertaining the need for operative intervention. Again, fluid in the pericardium immediately following penetrating trauma must be presumed to be blood. Of note, FAST false negatives may occur if the pericardial injury allows drainage into the hemithorax. Residual hemothorax or high-volume thoracostomy tube drainage suggests the need for SPW to further evaluate for missed cardiac injury. Transdiaphragmatic pericardial window for diagnosis or treatment can also be performed at the time of laparotomy for other injuries when indicated.
Pericardiocentesis does not have a role in penetrating thoracic trauma, with few exceptions. Despite medical literature suggesting that pericardial blood does not clot, in practical experience, this is not the case. Pericardial drainage by needle and catheter not only inadequately drains the pericardial sac but is prone to clotting once in place. From a diagnostic standpoint, FAST is more accurate (significantly higher sensitivity and specificity [20]) and less prone to complications, and therapeutic management should be definitive as will be discussed in the following section. At best, pericardiocentesis should be considered a temporizing maneuver when no surgical intervention is possible.
20.1.5.1 Emergency Department Thoracotomy and Operative Cardiac Approach
Surgical approach will depend, in part, upon patient presentation. Patients who present in extremis generally require emergency department thoracotomy (EDT), also referred to as resuscitative thoracotomy. Patients with evidence of cardiac tamponade or severe hemorrhage who can tolerate transport from the trauma bay to the operating room will benefit from a median sternotomy. Patients with combined thoracoabdominal trauma in whom the predominant source of hemodynamic instability is unclear may require SPW in the OR before or during exploratory laparotomy and potentially median sternotomy. Patients with benign presentations despite high-risk injuries suggesting the possibility of cardiac injury merit FAST plus either echocardiography or potentially CT angiography. As mentioned previously, patients with hemopericardium on FAST require, at the minimum, SPW with drainage, and generally a median sternotomy to address the source of the bleeding. Leaving patients with undrained hemopericardium risks both sudden and profound clinical deterioration as well as posttraumatic constrictive pericarditis.
Strict indications and limits of EDT remain a controversial topic. Overall survival following EDT for penetrating trauma is 11 % in combined studies; however, penetrating cardiac injury survival is higher, at 31 % [21]. The American College of Surgeons Committee on Trauma guidelines emphasize that penetrating cardiac wounds have the best chance of survival when they arrive after “a short scene and transport time with witnessed or objectively measured physiologic parameters (signs of life).” This does not address hard criteria for futility of EDT. The Denver Health group in collaboration with the Western Trauma Association prospectively analyzed resuscitative thoractomies and found a survival benefit for penetrating injury patient who had undergone less than 15 min of CPR without return of signs of life. Asystolic patients should generally be pronounced unsurvivable with the exception of those patients with asystole and concomitant pericardial tamponade, who may be salvageable [22]. These data support previous analyses demonstrating that, especially for penetrating thoracic trauma, EDT following these criteria may preserve life and acceptable neurologic outcomes [23]. Most would argue that profound hypotension (systolic blood pressures in the 60–70 mmHg range) requires emergent resuscitative thoracotomy in the trauma bay as well.
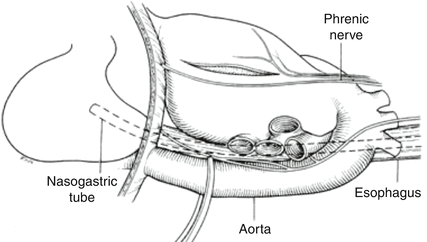
During resuscitative thoracotomy for cardiac injury, the primary goal is to open the pericardium to relieve potential tamponade and at least temporarily control massive hemorrhage. Pericardial clot may not be immediately visible; therefore, the pericardium should be rapidly opened in all patients. A knick in the pericardium is made with a scalpel anterior to the left phrenic nerve and then opened longitudinally with scissors or bluntly with a fingertip to avoid damage to the phrenic nerve (Fig. 20.3). As the heart is delivered from the pericardial sac and inspected for penetrating wounds, both anteriorly and posteriorly, fingertip pressure on injured areas should be applied. Insertion of a Foley catheter into gaping wounds with inflation of the balloon and gentle traction has been described to occlude the wound; however, this risks expanding the hole with any increase in tension. Atrial wounds may be occluded with a Satinsky clamp to allow a more controlled repair. Ventricular wounds may be sutured with a 2-0 or 3-0 nonabsorbable suture such as a nylon or polypropene; given the thinner wall of the right ventricle, Teflon pledgets may be helpful. Atrial wounds may be closed in a running fashion, while ventricular wounds are generally closed with an interrupted horizontal mattress, figure of eight, or simple sutures. Use of standard 6 mm skin staplers has also been described to temporarily control cardiac injuries with control of hemorrhage in 93 % of patients [24]. Wounds in direct proximity to major coronary vessels may require a horizontal mattress repair underneath the vessels. Additionally, if broader access is required in the emergency department, the left anterolateral thoracotomy may be extended to a bilateral anterior thoracosternotomy (“clamshell” incision) by crossing the sternum. The sternum may be divided with trauma shears, a Lebsche knife, or a Gigli saw. Note that if the patient recovers a perfusing blood pressure to allow further transport to the operating room, it is important to remember to ligate both ends of the bilateral internal mammary arteries that have, by necessity, been divided during this procedure.