Fig. 7.1
Three by three grid for recording quantitative description of pelvic organ support
Points and Landmarks for POP-Q System Examination
Table 7.1 illustrates the reference points: three reference points are anteriorly (Aa, Ba, and C) and three posteriorly (Ap, Bp, and D). Points Aa and Ap are 3 cm proximal to or above the hymenal ring anteriorly and posteriorly, respectively. Points Ba and Bp are defined as the lowest points of the prolapse between Aa anteriorly or Ap posteriorly and the vaginal apex. Anteriorly, the apex is point C (cervix), and posteriorly is point D (pouch of Douglas). In women after hysterectomy, point C is the vaginal cuff and point D is omitted. Three other measurements are taken: the total vaginal length (TVL) at rest, the genital hiatus (GH) from the middle of the urethral meatus to the posterior hymenal ring, and the perineal body (PB) from the posterior aspect of the genital hiatus to the mid-anal opening. Normal position of the reference points is illustrated in Fig. 7.2. Once the measurements are taken, the patients are assigned to a corresponding stage (Fig. 7.3).
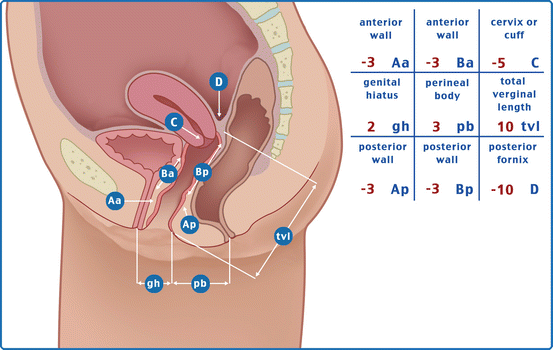
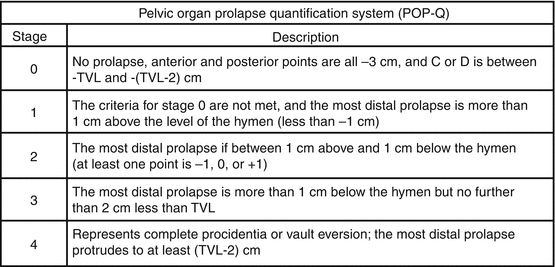
Table 7.1
Description of the points and their range of values
Points | Description | Range of values |
|---|---|---|
Aa | Anterior vaginal wall 3 cm proximal to the hymen | −3 cm to + 3 cm |
Ba | Most distal position of the remaining upper anterior vaginal wall | −3 cm to + tvl |
C | Most distal edge of cervix or vaginal cuff scar | −10 cm to + 10 cm |
D | Posterior fornix (N/A if post hysterectomy) | |
Ap | Posterior vaginal wall 3 cm proximal to the hymen | −3 cm to + 3 cm |
Bp | Most distal position of the remaining upper posterior vaginal wall | −3 cm to + tvl |

Fig. 7.2
Normal position of the reference points

Fig. 7.3
POP-Q staging
The POP-Q system has been criticized for being cumbersome and difficult to learn, although some instructional videotapes are available through the websites and facilitate the learning process. Some tips and tricks can be used to improve the system measurements. Different kinds of devices facilitate the examiner in making the measurements. Figures 7.4 and 7.5 show a home-made system in which a uterine dilatator is used to measure the point Aa (Fig. 7.4) and the point PB (Fig. 7.5). An alternative useful method in the clinical practice mark centimeters on the examiner’s finger and using it as a ruler (Fig. 7.6).
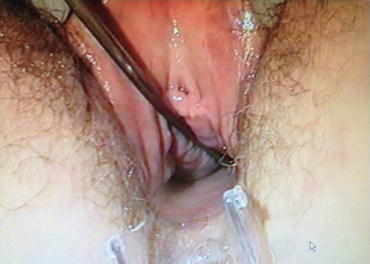
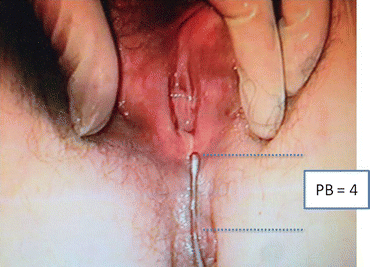


Fig. 7.4
Uterine dilatator is used to measure the point Aa

Fig. 7.5
Uterine dilatator is used to measure the point PB

Fig. 7.6
Finger with centimetric scale marked
It has been shown that the routine use of the POP-Q system decreases significantly the amount of time needed to collect the desired data. Experienced examiners averaged 2.05 min per examination while new examiners averaged 3.73 min. There is also a high correlation between the POP-Q findings in left lateral and lithotomy position.
Figures 7.7, 7.8, 7.9, 7.10, 7.11, 7.12, 7.13, and 7.14 illustrate some examples of the different stages of prolapsed anterior and posterior compartments, vault and uterus prolapse.
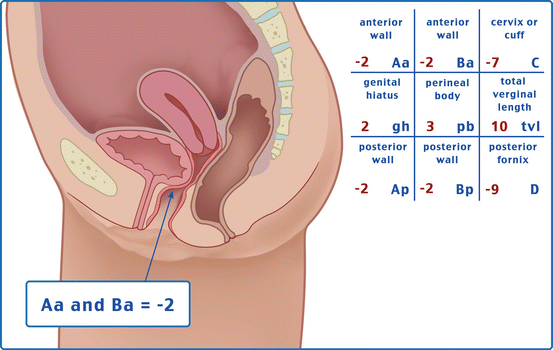
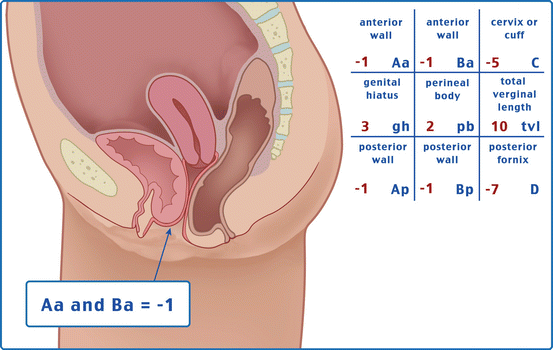
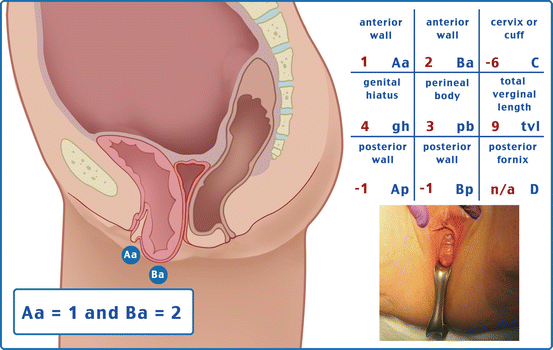
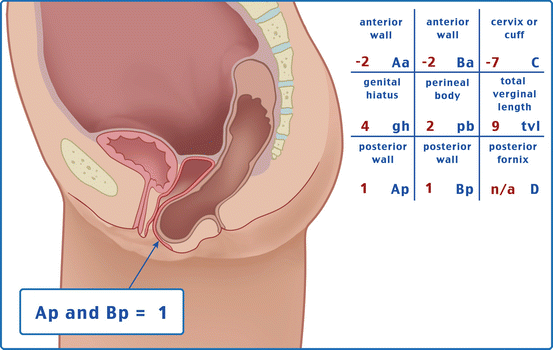
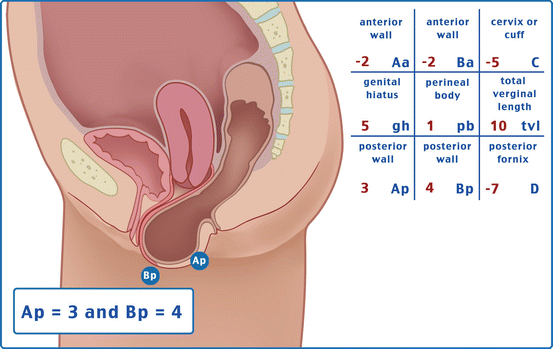
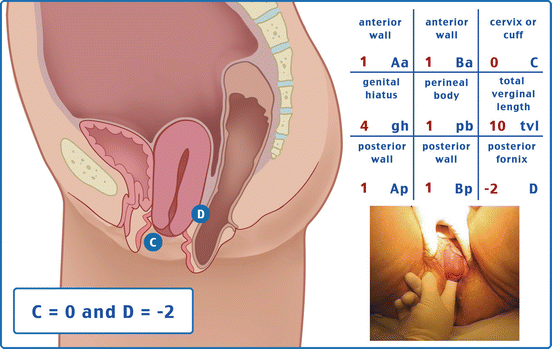
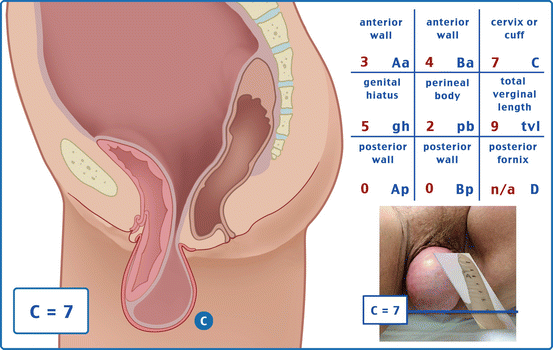
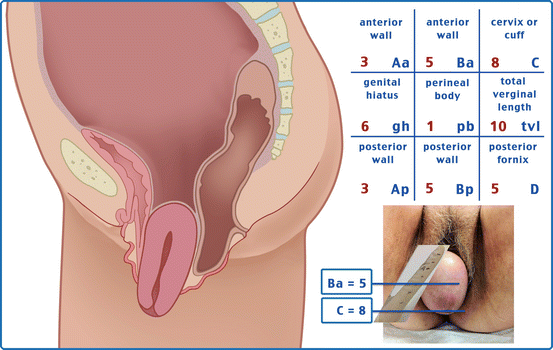

Fig. 7.7
Anterior prolapse stage 1 (with uterus)

Fig. 7.8
Anterior prolapse stage 2 (with uterus)

Fig. 7.9
Anterior prolapse stage 3 (without uterus)

Fig. 7.10
Posterior prolapse stage 2 (without uterus)

Fig. 7.11
Posterior prolapse stage 3 (with uterus)

Fig. 7.12
Uterine prolapse stage 2

Fig. 7.13
Vaginal vault prolapse stage 4

Fig. 7.14
Uterine prolapse stage 4
The POP-Q system does not include findings that some investigators believe to be essential for complete patient description, such as vaginal caliber, status of paravaginal sulci, pelvic muscle strength, or the presence of symptoms, although all of these information should be collected during POP evaluation.
Pelvic organ prolapse quantification (POP-Q) system is an objective, site-specific system for describing, quantifying, and staging pelvic support in women. It provides a standardized tool for documenting, comparing, and communicating clinical findings with proven interobserver and intraobserver reliability. It has been used for longitudinal follow-up of a population of women with prolapse and extensively for outcome reporting after prolapse repairs.
Recently a POP-Q simplified system has been developed, based on POP-Q with similar ordinal staging but with only four points measured instead of nine (Aa, Ba, C, D). Evaluation of the interobserver reproductibility and intersystems reliability (in comparison with the standard POP-Q system) showed good correlation.
Pessaries
(5)
Urology and Andrology Clinic, Department of Surgical and Biomedical Science, University of Perugia, Rome, Italy
Pelvic organ prolapse (POP) is common and is seen in up to 50 % of parous women in a clinic setting. In the general population, an estimated 30 % of women will have signs of prolapse although the majority are asymptomatic. The etiology of pelvic organ prolapse is complex and multifactorial. Risk factors include pregnancy, childbirth, congenital or acquired connective tissue abnormalities, denervation or weakness of the pelvic floor, aging, menopause, and factors associated with chronically raised intra-abdominal pressure such as obesity, cough, and heavy lifting. Women with prolapse may have a variety of pelvic floor symptoms. Only some of the symptoms are directly related to the prolapse, including pelvic heaviness, a dragging sensation in the vagina, a bulge, lump or protrusion coming down from the vagina, and backache. Symptoms of bladder, bowel, or sexual dysfunction are frequently present. Symptoms may negatively affect body image, quality of life, and a woman’s ability to perform everyday activities. Prolapse treatment may be dependent on a number of factors including the severity of prolapse, the bothersomeness of the associated symptoms, the woman’s general health, and the woman’s treatment preference. Options available for treatment are conservative (pelvic floor muscle training), mechanical support (such as vaginal pessaries), estrogens, and surgery. Before the nineteenth century, the primary POP treatment was the vaginal pessary and Fig. 7.15 shows some of spiral-, oval-, and doughnut-shaped pessaries used for prolapse during the eighteenth century. Despite numerous technological breakthroughs in the medical field over recent decades, pessaries have remained essentially unchanged throughout the twentieth century. The use of pessaries has become commonplace over many years without full evaluation of their efficacy in comparison to other modes of treatment such as surgery, estrogens, or pelvic floor muscle training. Eighty-seven to 98 % of clinicians report using pessaries in their clinical practice and 77 % of gynecologist report using pessaries as the first-line treatment for prolapse.


Fig. 7.15
Pessaries in the nineteenth century from Stromayr C: Die Handschrift des Schnitt-und Augenarztes Caspar Stromayr. Berlin, 1925
In 2013 the Cochrane review demonstrated a lack of consensus on the use of different types of the device, the indications, and the pattern of replacement and follow-up care. However pessaries are commonly used when conservative treatment, like physiotherapy, and surgery have either failed or are not suitable. They present a good option for patients who have not completed childbearing, do not desire surgery, or are poor surgical candidates.
The pessary is inserted into the vagina in order to physically support the vaginal walls, holding the prolapsed organs inside the vagina, supporting the pelvic structures and relieving pressure on the bladder and bowel. In this way it should be able to prevent the prolapse becoming worse and in some cases it is used to avert or delay the need for surgery.
Pessaries are now generally made of an inert plastic or silicone material to prevent odors and absorption of vaginal secretions. Silicone pessaries can be autoclaved and latex allergy is not a contraindication. There are currently many shapes and sizes of pessaries available to suit individual needs, all with their own advantages and disadvantages.
The clinicians choose the type of pessary based on severity of prolapse, presence or absence of the uterus, sexual activity, and concomitant stress urinary incontinence. A cystocele is best treated with a ring with support (filled-in center), uterine prolapse with a ring without support (hollow), and stress incontinence with a ring with a knob. Two main groups of pessaries are available: support pessaries and space-filling pessaries (Fig. 7.16). Generally the first-line pessary for clinicians is the ring pessary, due to ease of insertion and removal. The ring is easy to insert by folding the ring in half and placing a small amount of lubricant on the tip of the pessary to aid in insertion (Fig. 7.17). It is then placed into the vagina where it unfolds once above the pubic symphysis. For removal, the pessary is gently pulled and folded in half. A string can be attached to the ring to aid in insertion and removal. Patients can easily be taught to do this by themselves.
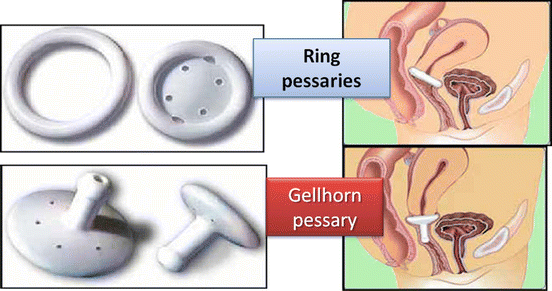
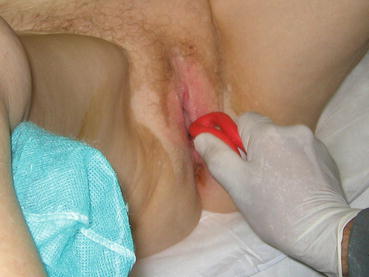

Fig. 7.16
Different pessaries and how they fit

Fig. 7.17
Insertion of a ring pessary
Another kind of pessary is the Gellhorn, generally used for more advanced-stage prolapse or in a patient who is no longer sexually active. It is a space-occupying pessary; its removal and insertion is more difficult and therefore cannot be done by the patient. This pessary has a concave portion attached to a stem that faces into the vagina (Fig. 7.16). To insert the Gellhorn, the pessary is folded in half with the use of lubricant on the leading edge to ease insertion. Once the pessary is behind the pubic symphysis, it will expand and rest against the leading edge of prolapse creating suction. To remove the Gellhorn, the knob is grasped, generally with the help of a ring forceps, while the concave end of the pessary is rotated to release the suction and the pessary is pulled downward, folded, and removed. Another space-filling pessary is the cube pessary. It is made of flexible silicone and is an option in cases of stage III and IV prolapse. The pessary has a string on one end for ease of removal. To insert, the cube pessary is compressed and inserted into the vagina. The cube applies suction to the leading edge of prolapse and often vaginal secretions are trapped in the crevices of the pessary, leading to malodorous discharge; it is usually the pessary of last resort. This pessary should be removed on a nightly basis when possible.
For pessary placement the patient should empty her bladder before pessary fitting. To begin fitting, the clinician can estimate the width of the mid-vagina and use this information to select the appropriate size pessary. The patient should be fitted with the largest size pessary that fits comfortably. The patient is examined in supine and standing position with and without Valsalva. The examiner should be able to comfortably fit a finger on either side of the pessary. If atrophy is present, estrogen should also be prescribed, generally in cream, ring, or tablet form. The patient is then instructed to ambulate, sit on the toilet, and strain to further assess comfort and appropriate pessary fitting. Once the correct pessary type and size are chosen and successfully fitted in place, the patient should attempt to remove and reinsert the pessary on her own. This is commonly possible with the ring type. It is always reassuring if the patient can void with the pessary in place before she leaves the office. Patients should be warned that urine leakage may increase with prolapse reduction. Pessaries can be removed daily, weekly, or monthly, at patients’ discretion, for washing with regular soap and water. Ring pessaries can be removed or left in place for intercourse. If patients are unable to care for the devices on their own, they will require health-care providers to remove, wash, and reinsert them every 3–6 months. To prevent infections and odors, an acidifier (usually supplied with the pessary) or estrogen must be applied vaginally 2 or 3 times a week. Oral or transdermal estrogen and an estradiol-17 ring (placed behind the pessary) are also options. All patients wearing pessaries should be examined by health-care professionals every 3–6 months to check for vaginal erosions or ulcers. If lesions are found, pessaries should be removed until the lesions have healed, and affected areas should be treated with topical estrogen.
There are very few contraindications to pessary use, which allows clinicians to offer pessaries to almost all patients presenting with prolapse and incontinence. Pessaries should not be placed in patients with evidence of an active pelvic infection or severe ulceration or allergy to silicone or those patients who are noncompliant and unlikely to comply with follow up. Risks of wearing a pessary include vaginal infection, erosions, discharge, odor, pain, bleeding, failure to reduce the prolapse, and expulsion. Serious complications from pessaries are rare; however, vesicovaginal fistula, rectovaginal fistula, erosion, and subsequent impaction have all been reported.
In conclusion the use of pessary is one possible option for conservative POP treatment. It is cheap and complications are reported to be rare; however the efficacy of pessary use in the management of prolapse still requires to be clearly established. In the 2013 Cochrane review, one randomized controlled trial compared ring and Gellhorn pessaries and the results showed that both pessaries were effective for approximately 60 % of women who completed the study with no significant differences identified between the two types of pessary. So far there is little evidence and no consensus on the indications, choice of device, or follow-up for pessaries. Although serious side effects are infrequent, insertion and removal of most pessary types still pose a challenge for many patients. Pessary design should continue to improve, making its use a more attractive option.
Anterior Repair
(6)
Urology and Andrology Clinic, Department of Surgical and Biomedical Science, University of Perugia, Perugia, Italy
(7)
Department of Urogynecology, San Carlo-IDI Hospital, Rome, Italy
Background
Anterior compartment support depends on the connection of the vagina and periurethral tissues to the muscles and fascia of the pelvic wall via the arcus tendineus fascia pelvis. On both sides of the pelvis, the arcus tendineus fascia pelvis is a band of connective tissue attached at one end to the lower sixth of the pubic bone, 1 cm lateral to the midline, and at the other end to the ischium, just above the spine (Fig. 7.18).
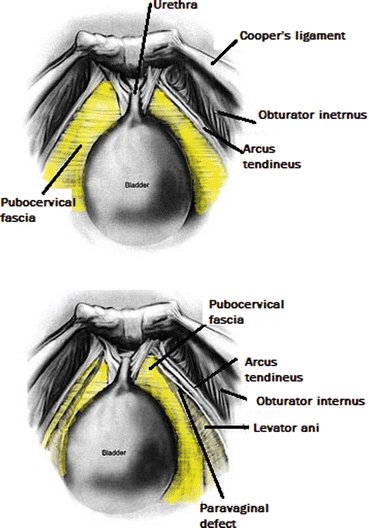

Fig. 7.18
Scheme of anterior compartment support
Anterior vaginal prolapse can result from defects in different areas of pelvic support including:
1.
Defects of the vaginal wall in the midline that result in “distension cystocele”
2.
Loss of the lateral attachment of the anterior vaginal wall to the pelvic side wall, referred to as a ‘”displacement cystocele”
3.
Loss of bladder neck support (urethrocele)
4.
Separation of the cardinal–uterosacral ligament complex from the vaginal apex (superior defect)
The goal of surgery is to repair these defects to recreate a strong anatomical support of the anterior vaginal wall.
Clinical Practice
Surgical correction of anterior vaginal prolapse should be proposed in case of symptomatic ≥2 stage cystocele.
Patients could complain of symptoms directly related to the prolapse: vaginal bulging and heaviness in the suprapubic area and/or pelvis; vaginal bleeding, discharge, or infection related to ulceration of the prolapse; need of digitally replace the prolapse to assist voiding; and low backache. Often patients refer also symptoms of bladder dysfunction and in particular storage symptoms (increased daytime frequency, urgency, nocturia), voiding symptoms (hesitancy, slow stream, feeling of incomplete emptying), urinary incontinence (stress, urgency or mixed incontinence), and sexual difficulty.
Diagnostic Evaluation
After a detailed history of bladder, bowel, and sexual function, a pelvic examination should be performed with patient in lithotomy position. A detailed assessment of the support of all segments of the vagina should be made using the pelvic organ prolapse quantification (POP-Q) system and an assessment for central, lateral, and superior anterior vaginal support. If physical findings do not correspond to symptoms or if the maximum extent of the prolapse cannot confirmed, the patient should be examined in standing position.
Urodynamics is useful to assess symptoms of urinary incontinence and voiding dysfunction that are common in women with advanced vaginal prolapse. Additionally, because significant anterior vaginal prolapse often results in kinking of the urethra that may mask underlying stress incontinence, a preoperative urodynamics with vaginal packing or pessary is recommended, to evaluate the lower urinary tract in these patients.
Magnetic Resonance Imaging (MRI) can be useful to further define the nature of the prolapse and evaluate for potential enterocele, rectocele, or uterine prolapse.
Preoperative cystoscopy is useful in the evaluation of the prolapse in patients with lower urinary tract symptoms such as urinary urgency, hematuria, and obstructed voiding to rule out concurrent bladder pathology.
Surgical Technique
Anterior vaginal prolapse resulting from a central defect is traditionally corrected with anterior colporrhaphy. Anterior colporrhaphy was popularized by Howard Kelly in 1911 and still remains the most common technique for transvaginal correction of the anterior vaginal prolapse. Recently to improve the outcomes and avoid the recurrences, the anterior colporrhaphy with use of graft has been proposed. On July 13, 2011, the FDA issued a statement that serious complications are not rare with the use of surgical mesh in transvaginal repair of pelvic organ prolapse. For this reason and on the basis of the Cochrane review conclusions that the transvaginal repair of pelvic organ prolapse with mesh does not improve symptoms or quality of life more than non-mesh repair, the use of mesh during transvaginal surgery is recommended only in the context of RCTs.
Although many variations of anterior repair technique have been described in the last century, the basic approach is still similar to that originally described by Kelly.
A longitudinal mucosal incision is made near the apex of the vagina (Figs. 7.19 and 7.20) and, through this, the vesicovaginal space entered with the tip of a pair of Metzenbaum scissors. Dissection is then performed bluntly by opening and closing the scissors just beneath the vaginal mucosa. The mucosa is incised and the dissection continues along the length of the vaginal wall to within 1 cm of the urethral meatus. Sharp and blunt dissection are used to mobilize connective tissue (vesicovaginal fascia), bladder, and urethra away from the overlying epithelium, just beneath the mucosa. Thompson speculated that the success of the procedure depends on the integrity of the fascial layer left attached to the bladder. Later dissection into the space beneath the inferior pubic ramus is necessary bilaterally (Fig. 7.21). Kelly plication is then performed: a tissue bite is taken laterally in the periurethral tissue just under the symphysis pubis, with the suture crossing under the bladder neck, before a similar bite is taken on the other side. Then, three to five delayed absorbable sutures are placed to reduce the cystocele (Figs. 7.22, 7.23, and 7.24). Care must be taken to avoid deep tissue bites, as such bites may result in ureteral kinking. Then excess vaginal mucosa is trimmed bilaterally (Fig. 7.23) and the anterior vaginal wall is closed with interrupted vertical mattress sutures that include the underlying connective tissue. A vaginal pack can be placed for 24 h to minimize the chance of postoperative hematoma formation. Cystoscopy should then be performed to ensure bladder and ureteral integrity.
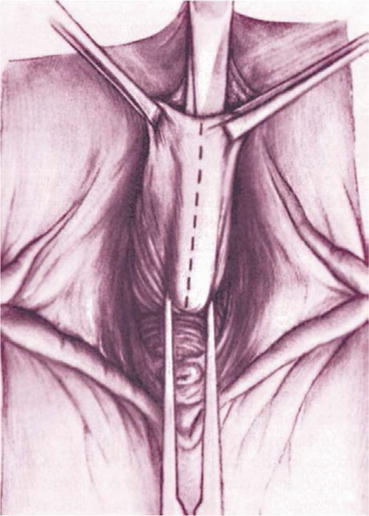
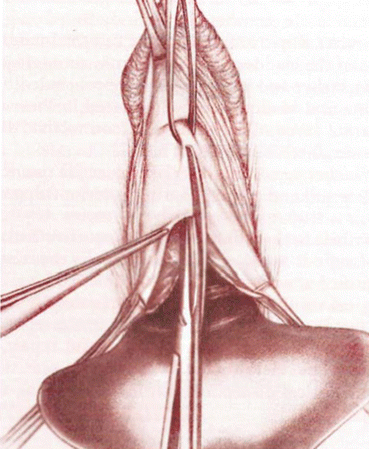
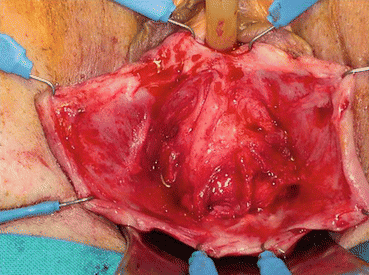
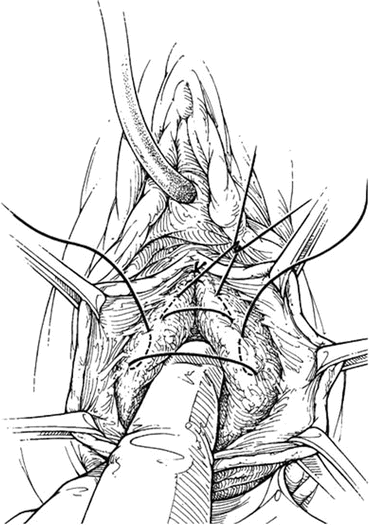
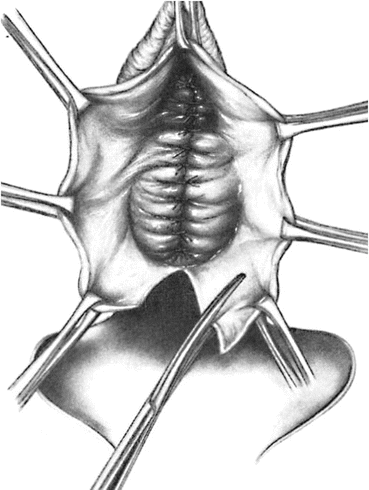
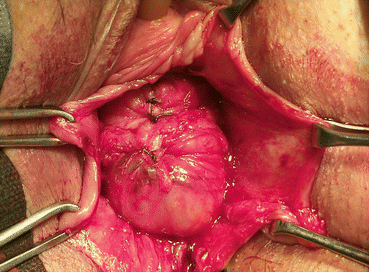

Fig. 7.19
Anterior midline incision

Fig. 7.20
The vaginal mucosa is incised to enter the vesicovaginal space

Fig. 7.21
Surgical view: the bladder is isolated and dissected from the vaginal wall mucosa

Fig. 7.22
Delayed absorbable sutures are placed on the lateral edges of the fascia to reduce the cystocele

Fig. 7.23
Colporrhaphy is completed and the excess vaginal mucosa is trimmed of bilaterally

Fig. 7.24
Surgical view of Anterior repair sutures
When anterior vaginal prolapse results from a lateral detachment of the anterior vaginal wall at the pelvic side wall, the goal of surgery is to reattach the lateral vaginal sulcus to its normal lateral attachment. The lateral vaginal attaches to the levator ani (LA) muscle along a line from the anterior pubic rami to the ischial spine known as the “white line” or arcus tendineus fascia pelvis (ATFP) (Fig. 7.18). The paravaginal defect can be repaired retropubically or vaginally. The transvaginal approach (vaginal paravaginal repair) can be more challenging than the retropubic approach but has the advantage of avoiding an abdominal incision and facilitating a concurrent central defect. In case of transvaginal approach, the vaginal epithelium is sharply dissected from the underlying vaginal muscularis and the dissection is continued laterally to the ATFP. Four to six interrupted nonabsorbable sutures are placed through the ATFP and the aponeurosis of the LA muscle at 1 cm intervals. Each stitch is then placed through the lateral edge of the pubocervical fascia and then tightened.
Complications
Accidental cystotomy, particularly in women with previous vaginal surgery. In this case, the defect is closed in two layer using delayed absorbable sutures, and the bladder is drained for 5–7 days to allow for adequate healing.
Ureteric injury, particularly during placement of the Kelly plication sutures. To avoid this complication, the distance between the sutures and the ureteric orifices must be greater than 0.9 mm.
Voiding symptoms (greater than 3 months), which occurs more often in women with some degree of preoperative voiding dysfunction as suggested by preoperative urodynamic parameters (elevated post-void residual >100 ml, decreased peak voiding flow rate <15 ml/s, and low voiding detrusor pressure >15 cmH2O). Long-term voiding difficulty is best managed with clean intermittent self-catheterization.
Detrusor overactivity, which could be due to the surgical dissection around the urethra and bladder neck that damages the parasympathetic and sympathetic nerves leading to denervation of the urethra and detrusor muscle.
De novo stress incontinence, which was attributed to a hypercorrection of the cystocele with a loss in the normal posterior urethrovesical angle between the bladder base and the bladder neck. Currently it is believed that de novo stress incontinence could be due to the denervation of the urethral sphincter or to a condition of latent stress incontinence that is unmasked when urethral kinking is surgically corrected.
Decreased mobility and capacity of the vagina with subsequent dyspareunia and sexual dysfunctions (avoid to remove the vaginal walls in excess).
Regarding vaginal paravaginal repair, significant complications have been reported such as ureteric obstruction, retropubic hematoma, vaginal abscesses, and abnormal bleeding.
What Do the Guidelines Say
Outcomes of the traditional surgery for the correction of the anterior vaginal prolapse are largely limited to retrospective reviews and case series. Moreover, reporting of outcomes has been extremely subjective and, before the advent of POP-Q system, pre- and postoperative staging have been quite variable between surgeons.
Considering these limitations, the success rates of anterior colporrhaphy ranged from 80 to 100 % in retrospective series, while the success rates of the vaginal paravaginal repair for cystoceles vary from 67 to 100 % in case series. Studies that differentiated lateral from central recurrences have revealed that central recurrence (22–25 %) is more common than a lateral recurrence (2–8 %).
Comparing anterior colporrhaphy alone to anterior repair with graft or mesh reinforcement, it was demonstrated that absorbable polyglactin mesh (Vicryl) might reduce objective prolapse recurrence and this is true also using absorbable porcine dermis or polypropylene mesh. However improved outcomes including patient satisfaction, quality of life, and reduced operations for recurrences have not yet been demonstrated. Furthermore, anterior polypropylene mesh alone demonstrated an improved subjective outcome as compared to native tissue anterior repair, but there was no difference between the groups in the rate of dyspareunia. The operating time, blood loss, rate of apical or posterior compartment prolapse, and de novo stress urinary incontinence were greater in the polypropylene mesh group, which was associated with an 11.4 % rate of mesh erosion and 6.8 % requiring surgical reintervention.
Sacrospinous Fixation
(8)
Urogynecology Unit, Department of Gynecology, San Carlo-IDI Hospital, Rome, Italy
(9)
Urology and Andrology Clinic, Department of Surgical and Biomedical Sciences, University of Perugia, Perugia, Italy
Background
Post-hysterectomy vault prolapse and uterovaginal prolapse are challenging problems for the pelvic reconstructive surgeon. The main cause of these apical prolapses is the weakness of the uterosacral/cardinal ligament complex, and the restoration of this support is the “cornerstone” of the reconstructive surgery. Various approaches are available to achieve this: vaginal and open, or laparoscopic techniques. The vaginal approach includes: the iliococcygeus fascia fixation, the uterosacral ligament suspension, the Mayo/McCall culdoplasty, and the sacrospinous fixation (SSF).
Clinical Practice
The SSF gained popularity in the second half of the twentieth century, and its most common indication is the resuspension of a symptomatic, post-hysterectomy prolapsed vaginal apex.
Sacrospinous fixation is also indicated when women desire to preserve fertility or the uterus. SSF may also be used to decrease the operative time and morbidity in elderly women who have no evidence of uterine pathologies.
Diagnostic Evaluation
After a detailed history, a pelvic examination should be performed with the patient in the lithotomy position using the pelvic organ prolapse quantification (POP-Q) system. In cases of post-hysterectomy vaginal vault prolapse, a simultaneous rectal and vaginal examination may aid in detecting the presence of an enterocele and in differentiating an enterocele from a high rectocele. Many radiologic modalities have been used in the diagnosis, including defecography, fluoroscopy, dynamic cystodefecography, and MRI. In cases of severe uterovaginal prolapse of the anterior and apical vaginal wall, the upper urinary tract must be evaluated because severe prolapse can cause angulation of the ureters resulting in ureteral obstruction.
Surgical Technique
Several modifications of the SSF have been described. The original technique described by Nichols involves suspension of the vaginal vault to the sacrospinous ligament (SSL) (Fig. 7.25) through the posterior compartment (Table 7.2). A modification was introduced by Winkler et al., suspending the vault to the SSL through the anterior compartment, with the aim to reduce the postoperative narrowing and lateral deviation of the upper vaginal wall. In 1993 Kovac and Cruikshank proposed the sacrospinous hysteropexy, in which the uterus was suspended bilaterally to the SSLs.
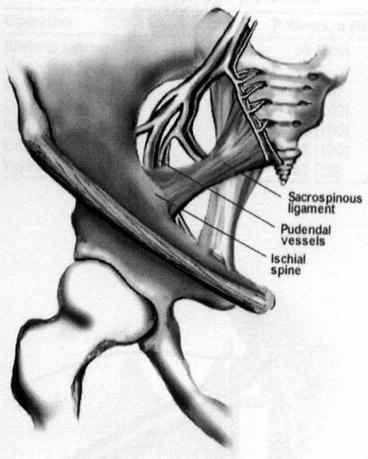

Fig. 7.25
The sacrospinous ligament and its relation with pudendal vessels and nerves and ischial spine
Table 7.2
The technique step-by-step
Identification of the pararectal space |
Visualization of the sacrospinous ligament |
Avoid injury of pudendal vessel and nerve (behind the ligament and the ischial spine) |
Sutures >2 cm medial to the spine |
Nonabsorbable sutures |
SSF is typically performed unilaterally on the right side (Fig. 7.26), which offers the anatomical advantage of absence of the sigmoid colon on this side. In cases of severe genital prolapse, bilateral suspension may be considered. In this latter case the surgeon must ensure that there is adequate vaginal space, so that the bilateral suspension does not cause stricture in the rectum.
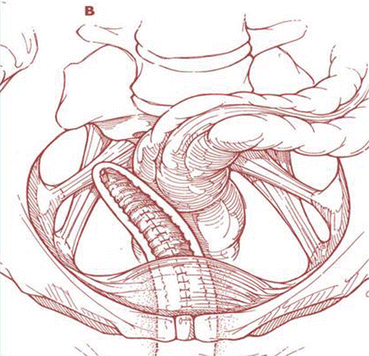

Fig. 7.26
Schematic view of monolateral sacrospinous ligament fixation
The posterior approach involves a posterior vaginal incision and a posterior colporrhaphy dissection, with subsequent perforation of the rectal pillar near the ischial spine. With blunt dissection of the pararectal space medial to the ligament, the coccygeus muscle and the sacrospinous ligament are exposed (Fig. 7.27). The anterior approach involves an anterior vaginal wall incision and a dissection of the ipsilateral paravesical and paravaginal area from the level of the bladder neck to the ischial spine along the arcus tendineus fasciae pelvis (Fig. 7.28). This dissection opens a large space for the vaginal apex and avoids the narrowing often caused by the posterior approach. Then the ligament is exposed with posterolateral dissection within the retroperitoneal place using two Breisky-Navratil retractors (one placed medially to sweep the rectum medially off the ligament and the other laterally) and a Haney retractor posteriorly, just in front of the coccygeus muscle at the 7 o’clock position. Various devices (Fig. 7.29) have been used to place sutures into the sacrospinous ligament, including the Deschamps ligature carrier, Miya hook, and Capio in-line “push and catch” suturing devices. The lateral suspension suture is placed through the ligament 1–2 cm medially to the ischial spine to avoid injury to the pudendal vessels. Fixation of the vaginal apex is most commonly performed with permanent monofilament sutures (Fig. 7.30), but some surgeons prefer absorbable sutures to reduce the risk of suture erosion and granuloma formation. The relative advantages of permanent versus absorbable sutures have not been evaluated.
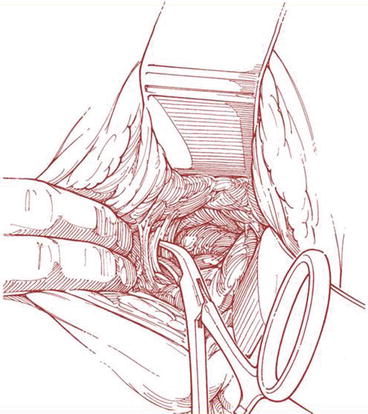
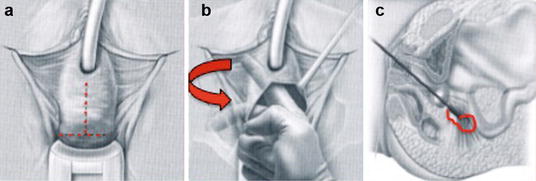
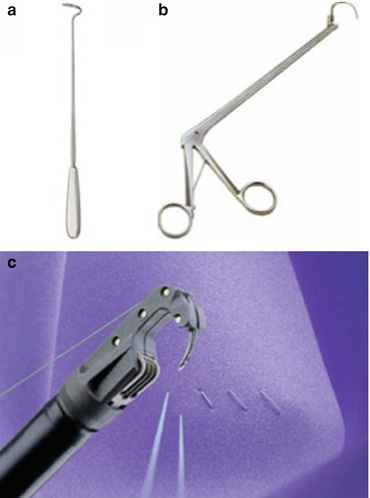
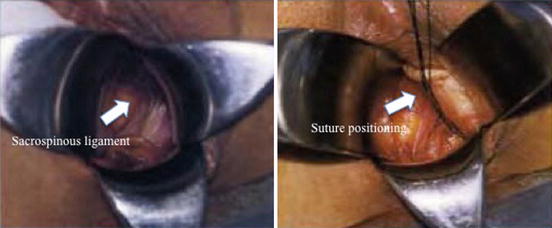

Fig. 7.27
Exposure of sacrospinous ligament

Fig. 7.28
Sacrospinous ligament fixation – anterior access: (a) anterior vaginal wall incision, (b) dissection of paravesical and paravaginal area, (c) placement of suture into the sacrospinous ligament

Fig. 7.29
Devices used to place sutures into the sacrospinous ligament – (a) Deschamps ligature carrier, (b) Miya hook, (c) Capio™ suturing device

Fig. 7.30
Sacrospinous ligament fixation: intraoperative images
In cases of uterine hysteropexy, the dissection and suture placement are similar to those of vault suspension. The difference is that, after suture placement in the sacrospinous ligament, the end of the suture is brought through the uterosacral ligament and then through the vaginal epithelium.
Complications
Local neurologic complications may include pudendal, sacral, or sciatic neuropathy. A direct injury to major nerves is rare. In this case a re-operation is mandatory to remove the sutures. More frequently pudendal nerve entrapment occurs and results in pain localized in the buttock or perineum. The pain may improve after replacement of lateral fixation sutures more medially. Moreover, gluteal pain or paresthesias occurs after SSF, possibly due to peripheral nerve trauma. Usually these symptoms are transient and self-limited; sometimes they persist for weeks or months postoperatively.
Serious vascular injury is a rare complication, but it can be life-threatening. It usually results from injury of small vessels along the medial aspect of the rectum. Less commonly, the medial retractor can disrupt deeper vessels in the presacral area. For most cases, sutures or surgical clips to the involved vessels can be placed under direct vision after exposure with simple retraction. If the vessels cannot be visualized, the use of prolonged pressure packing may become necessary. Rarely a selective arterial embolization is necessary.
More rare complications of SSF include: infections, suture abscesses, bowel injury, and bladder lacerations.
Point of Interest
The anatomical cure rate of the apical vaginal segment after SSF ranges from 89 to 90 %, but the anterior vaginal segment represents a site particularly vulnerable to recurrence. Recurrent cystoceles have been reported in 7.6–92 % of patients after SSF, depending on the other concomitant procedures performed. It has been proposed that posterior deflection of the vaginal wall after SSF leads to increased exposure of the anterior compartment to intraperitoneal forces. It has also been suggested that underestimation of the severity of anterior prolapse on preoperative examination may play a role. A preoperative identification of all areas of prolapse and a subsequent modification of the pelvic reconstruction technique according to intraoperative findings are important key elements to reduce this kind of complication.
Sexual dysfunction has been reported after SSF. The retroversion of the vaginal apex and deviation after unilateral SSF appear to have no adverse effects on coital function, but the postoperative vaginal narrowing is likely correlated with this kind of dysfunction. Some papers underlined that vaginal narrowing after SSF was more likely the result of repair of other concurrent vaginal defects (mainly anterior colporrhaphy), rather than of SSF itself.
What Do the Guidelines Say
Abdominal Sacral Colpopexy Versus Vaginal Sacrospinous Colpopexy
SSF showed a higher rate of recurrent vault prolapse, more dyspareunia, shorter operating time and recovery time, and lower cost. Data on the subjective success rate, patient satisfaction, and impact of the surgery on quality of life were too few for reliable conclusions.
Comparing Different Vaginal Approaches: One Type of Upper Vaginal Prolapse (Uterine and Vaginal Vault) Repair Versus Another
The last Cochrane review on POP surgery (2013) concluded that all vaginal approaches to correct upper vaginal prolapse, i.e., uterosacral ligament suspension, McCall culdoplasty, iliococcygeus fixation, and colpocleisis, are relatively safe and effective interventions (level 3 evidence, grade C), but there is no evidence that one technique is better than another.
Vaginal Sacrospinous Uterine Suspension Versus Vaginal Hysterectomy
Women undergoing sacrospinous hysteropexy had a median hospital stay that was 1 day shorter than that in the hysterectomy group, and the mean number of days to return to work was 23 days less compared to the hysterectomy group. No differences were reported in domain scores for quality of life and urogenital symptoms between the two procedures. There were more adverse symptoms in the SSF group, mostly due to buttock pain. No differences were found between the two groups in terms of apical, anterior, and posterior prolapse recurrences.
Total Vaginal Polypropylene Mesh (TVM) Versus Sacrospinous Colpopexy
The SSF group had a higher objective recurrence rate. No differences were identified between the groups in terms of de novo SUI, bladder overactivity, dyspareunia, and pelvic pain or in functional outcomes measured with the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), the Urinary Impact Questionnaire (UIQ), the Colo-Recto-Anal Impact Questionnaire (CRAIQ), or the Pelvic Organ Prolapse Impact Questionnaire (POPIQ).
Meshes
(10)
Urogynecology Unit, San Carlo-IDI Hospital, Rome, Italy
(11)
Urology and Andrology Clinic, Department of Surgical and Biomedical Science, University of Perugia, Rome, Italy
Background
Despite modifications and evolutions of the technique, traditional surgery for pelvic organ prolapse (POP) has a failure or recurrence rate of 30–50 % and a prevalence of reoperation of 29–40 % within 3 years.
The highest rates of reoperation occur for apical defects (33 %), combined anterior and apical defects (15 %), or posterior and apical defects (12 %).
In an effort to improve outcomes in POP repair, especially of the anterior compartment, multiple biological and synthetic graft materials have been introduced.
Prosthetic materials have at least four main purposes in pelvic reconstructive surgery:
Substitution, where lacking supportive tissue is replaced by mesh
Reinforcement, where inadequate tissue is augmented to improve its performance
Generation, which involves the induction of new supportive tissue
Consolidation, where mesh is used to complement surgery
On the basis of these premises, the rationale for the use of mesh is:
To replace the damaged visceral fascia
To restore cohesion between visceral and parietal fascia to rebuild the hammock connected to the arcus tendineus fascia pelvis
To stabilize the bladder hammock
Unfortunately, because of secondary complications associated with the use of mesh, all the guidelines and reviews recommend their use under strict control. In particular the evidence is not sufficient to support the use of permanent meshes or grafts at the time of vaginal apical or anterior compartment repair surgery except in the context of randomized controlled trials.
Type of Meshes
The ideal graft/mesh should be:
Clinically and physically inert
Noncarcinogenic
Mechanically strong
Without allergic or inflammatory reaction
Sterile
Not physically modified by body tissue
Readily available
Inexpensive
With minimal risk of infection and rejection
The optimal implant, once healed, would restore normal anatomy and function to the vagina and the surrounding pelvic organs and be more durable or equally durable to autologous tissue. It would be biocompatible, and, if biodegradable, it should persist long enough for incorporation of the surrounding native tissue. It should resist to mechanical stress and shrinkage, be pliable and easily manipulated during surgery, and not result, once implanted, in adhesion formation at the visceral surfaces.
Currently, there are no biologic or synthetic implants that meet all the above criteria.
Surgical mesh materials can be divided into three general categories:
Synthetic
Biologic
Composite (i.e., a combination of any of the previous two categories)
Synthetic Meshes
Synthetic mesh can be absorbable or nonabsorbable. Once absorbable synthetics are implanted, macrophage activation leads to mesh absorption and subsequent recycling of by-products into new collagen fibers. The commercially available absorbable synthetic mesh implants are polyglycolic acid and polyglactin 910. Although both products are considered absorbable, their properties are distinct. Polyglactin 910 starts to hydrolyze during the third week after implantation and loses the majority of its mechanical value after 30 days, whereas polyglycolic acid requires 90 days for absorption.
The main characteristics of nonabsorbable synthetic meshes are pore size, fiber type, and stiffness, and on the basis of pore size and fiber type, they have been classified in four types (Fig. 7.31):
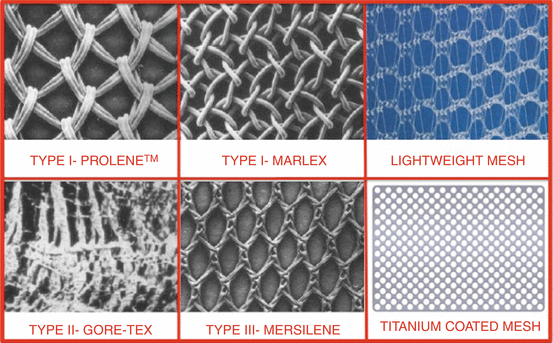

Fig. 7.31
Different synthetic meshes used in urogynecology
Type I meshes (Prolene®, Marlex®) contain large pores (>75 μm) that allow the admission of macrophages to prevent infection and in-growth of fibroblasts, blood vessels (angiogenesis), and collagen, to form fibrous connections to the surrounding tissue.
Type II meshes (Gore-Tex®) have a pore size <10 μm in at least one of the three dimensions (microporous).
Type III meshes (Mersilene®) are macroporous but have microporous components that often include braided and/or multifilament materials.
Type IV meshes have submicronic pore size.
Flexibility is an important property that appears to be related to pore size: the greater the pore size and the more the mesh flexibility, the lower the erosion rate. Currently the type I meshes are considered the best choice in urogynecology.
Recently other kinds of meshes have been introduced: the titanium-coated mesh, the ultralightweight mesh and the polyvinylidene fluoride (PVDF) monofilament mesh (Fig. 7.31). Some studies found that coating the mesh with titanium causes less severe inflammatory reactions than non-coated polypropylene. Ti-mesh® is a titanium-coated low-weight mesh and combines the advantages of light material with a superior biocompatibility due to the titanium coating, at least in terms of chronic inflammatory reactions.
Another mesh today available is the lightweight mesh, to add more favorable biocompatible characteristics without losing any critical tensile strength mesh. The main characteristics of lightweight meshes are:
Reduced overall “mesh load,” high hydrophilic
Low memory and easily intraoperative handling while maintaining durability
Generally not palpated during postoperative examinations
Different types are available in different shapes including Artisyn® Y-Shaped Mesh, Alyte® Y-Mesh Graft, and Restorelle™ Y.
The Y-meshes, particularly useful in colposacropexy, have a vaginal section with anterior and posterior flaps and a sacral section. The anterior and posterior flaps are used for anterior and posterior vaginal attachment, and the sacral flap for attachment to the sacral promontory (Fig. 7.32). Finally the polyvinylidene fluoride (PVDF) (DynaMesh®) mesh, widely used in abdominal hernia repair, has been recently introduced in the POP surgical treatment. It is highly biocompatible, it seems to be more resistant to hydrolysis and degradation in comparison to polyester and polypropylene. It also shows a reduced inflammatory and fibrotic reaction compared to polypropylene.
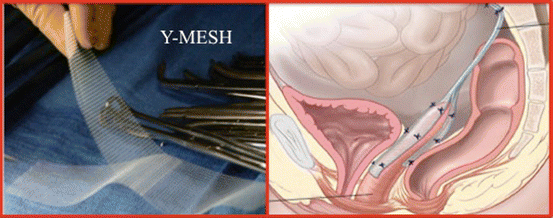

Fig. 7.32
Y-mesh used in colposacropexy. The anterior and posterior flaps are used for anterior and posterior vaginal attachment and the sacral flap for attachment to the sacral promontory
Up to now it is not clear whether the features of these new meshes would translate into any direct clinical benefit. Such questions can only be answered through clinical research.
Biologic Grafts
The most commonly used biologic materials available for POP repair include:
Autograft (rectus fascia)
Allograft (cadaveric fascia)
Xenograft
Xenografts may be either nonabsorbable (i.e., Pelvicol®) or absorbable (small intestine submucosa [SIS]) (Fig. 7.33). Pelvicol® is derived from porcine dermis: a patented process removes all fats and cellular materials through a series of organic and enzymatic extraction that leaves the material without any DNA. Then the matrix is stabilized using a cross-linking agent to maintain strength and provide permanence. Gamma sterilization of the tissue ensures sterility. It has been demonstrated that perforating the porcine dermis improves the graft take and decreases wound infections by allowing for increased tissue in-growth and revascularization of the vaginal epithelium overlying the graft. Tensile strength and suture pullout strength were maintained in the perforated graft.
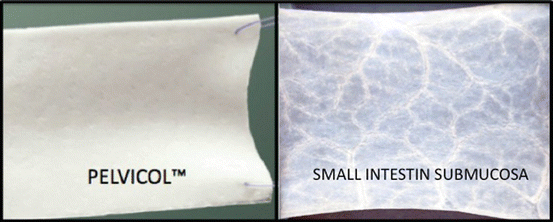

Fig. 7.33
Biological grafts
SIS is harvested from porcine jejunum and is composed of mucosa, muscularis mucosa, and submucosa. Processing leaves the extracellular collagen matrix intact, thus allowing the presence of collagen, growth factors, glycosaminoglycans, proteoglycans, and glycoproteins to promote host cells proliferation through SIS layers. Theoretically, the SIS scaffold should be entirely remodeled and replaced by the host’s connective tissue in 90 days.
Surgical Techniques Using Meshes
Historically the first mesh used in POP repair refers to abdominal colposacropexy, the gold standard for the surgical correction of vaginal vault prolapse, a technique which demonstrated a long-term durability and precise anatomical restoration. The goal of this procedure is to elevate the vaginal apex to the sacral promontory using a mesh bridge: the resultant vaginal axis is the most physiologic of all reconstructive procedures, and the vaginal length is maintained.
Colposacropexy can be also performed by laparoscopic- or robotic-assisted procedure with the aim to improve visualization of anatomy of the peritoneal cavity because of laparoscopic and robotic magnification; decrease postoperative pain; shorten hospitalization, resulting in potential cost reduction; and allow more rapid return to work.
In the last two decades, the meshes started to be used also in the vaginal approach to correct cystocele (anterior repair) and rectocele (posterior repair) and also the top of the vagina to correct uterine prolapse or vaginal apical prolapse (apical repair).
Vaginal Anterior Repair
Hand-Tailored Mesh
In the 1990s, urogynecologists began using surgical mesh for transvaginal POP repair and, to do so, surgeons cut the mesh to the desired shape, placing it through a corresponding incision. A variety of permanent polypropylene mesh overlays have been evaluated in case series for the management of anterior wall prolapse with an anatomical success rate ranging from 76 to 100 % (Fig. 7.34a).
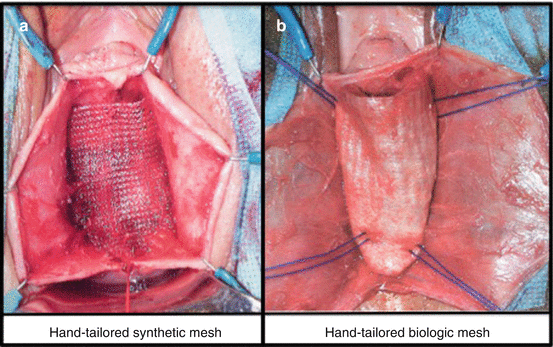

Fig. 7.34
Tailored synthetic (a) and biological grafts (b)
With the aim to reduce the complications associated with the synthetic meshes, biologic materials (cadaveric fascia lata, porcine dermis, porcine small intestine submucosa, bovine pericardium collagen) have been used (Fig. 7.34b). The reported success rate widely ranges from 52 %, using porcine dermis, to 100 % using cadaveric fascia lata.
Trans-obturator Kits
Over the next years, surgical meshes for transvaginal POP repair became incorporated into “kits” that included tools to aid in the delivery and insertion of the mesh. The surgical mesh kits continue to evolve, adding new insertion tools, tissue fixation anchors, and surgical techniques, using absorbable and biologic materials. The trans-obturator kits consist of four helical needles and a polypropylene mesh with four arms, two for each side. After the dissection of the bladder laterally toward the ischiopubic ramus, the superior needle is inserted lateral to the level of the clitoris, with an angle of 45° from the patient’s midline (Fig. 7.35a, b). Then the inferior needle is inserted 3 cm below and 2 cm lateral to the superior needle (Fig. 7.35c, d). The tip of the needle should be pointed directly at the ischial spine. The procedure is repeated on the contralateral side. The endoscopy is then performed to ensure the integrity of bladder and urethra. The mesh should now site underneath the cystocele in a tension-free manner, the tail is attached at the vault and under urethra, and the redundant tail is trimmed off. The vaginal incision is closed, the plastic sheaths are removed, and the mesh arms are cut at skin incisions.
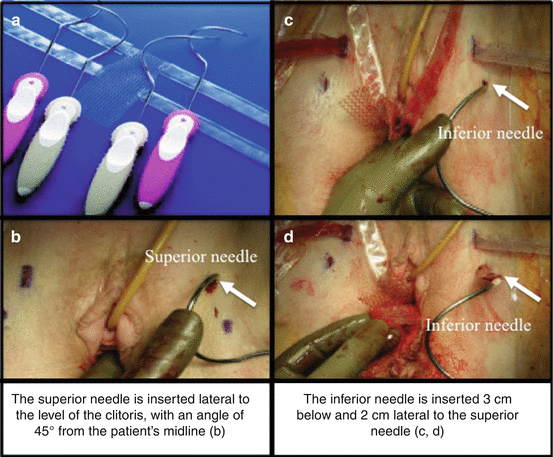

Fig. 7.35
Trans-obturator kits for anterior vaginal POP repair: (a) the elicoidal needles and the polypropylene mesh; (b) position and insertion of the superior needle; (c, d) insertion of the inferior needle
Single-Incision Kits
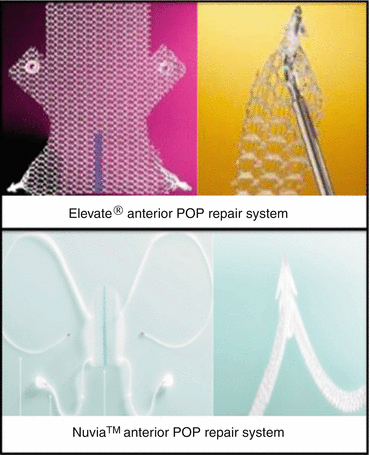
Recently new single-incision kits were introduced in the market. The devices allow a minimally invasive approach to treat anterior and apical defects. The mesh is inserted through a small vaginal incision and secured in their position using self-fixating tips attached to the mesh (Fig. 7.36); they are inserted into the sacrospinous ligament (SSL) and the obturator internus muscle without trocars to secure the correct positioning of the mesh until natural tissue ingrowth occurs.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








