Technological advances in imaging as well as increased knowledge of tumor-specific biology have promoted the role of organ-sparing approaches to pediatric renal and testicular tumors. Application of these techniques continues to evolve as data on long-term follow-up become available and as protocol-guided investigation provides answers to therapeutic outcomes of these approaches. Optimally, organ-sparing surgery will continue to provide increased potential for preservation of both renal function and fertility.
Nephron-sparing surgery
Partial Nephrectomy for Wilms Tumor: Introduction
Current accepted indications for renal-sparing surgery in the setting of Wilms tumor are bilateral Wilms tumor (BWT), Wilms tumor in a solitary kidney, and multicentric, unilateral Wilms tumor in patients at risk for metachronous tumors. Renal-sparing surgery in unilateral Wilms tumor in patients who are not at risk for metachronous tumors remains controversial. In this section, studies regarding partial nephrectomy in patients with BWT, solitary kidneys, or Wilms tumor-associated syndromes are reviewed. The current ongoing Children’s Oncology Group (COG) protocol for bilateral Wilms tumor is outlined and the current literature on nephron-sparing surgery in unilateral Wilms tumor patients is discussed. Finally, several surgical techniques for performing a partial nephrectomy in the setting of Wilms tumor are described.
Bilateral Wilms Tumor
For more than 20 years, the National Wilms Tumor Study Group (NWTSG) has recommended preoperative chemotherapy for patients with BWT, with the goal of avoiding total nephrectomy at initial surgery. In reviewing prior NWTSG trials, 9.1% of patients with BWT eventually developed renal failure; in almost three-fourths of these patients, the cause of renal failure was a contralateral nephrectomy for recurrent or persistent tumor after initial nephrectomy. BWT patients also have had significantly worse outcomes than similar patients with unilateral Wilms tumor. In NWTS-3 and -4, 344 patients with BWT had a 10-year relapse-free survival rate of only 65% and a 10-year overall survival rate of 78%, compared with unilateral stage I to IV favorable histology Wilms tumor (recurrence-free survival 86% and overall survival 92%). Poor outcomes in patients with BWT may have various contributing factors including delay in local control, understaging and undertreatment, or increased incidence of unfavorable histology (ie, >10% have anaplasia). Of the 145 patients enrolled in NWTS-2 and -3, total excision of tumor was possible in less than 40. In NWTS-4, complete removal of all gross tumor using a nephron-sparing approach was possible in 88% of kidneys, but local recurrence occurred in 8.2% of the remaining kidneys. In comparison, patients with unilateral tumors of favorable histology who underwent complete nephrectomy only had a 3% incidence of local recurrence. This higher rate of local recurrence in patients with bilateral tumors may be due in part to surgical technique; surgeons may accept a higher rate of positive margins to spare a normal kidney. More intensive up-front preoperative chemotherapy may reduce tumor burden in these patients, facilitating nephron-sparing surgery. The current COG protocol aims to improve outcomes in BWT patients while decreasing treatment-related morbidity.
Role of Initial Biopsy in Bilateral Tumors
Two reasons are typically given for performing preoperative biopsy. The first reason is to avoid misdiagnosis. In patients with bilateral renal lesions on imaging, misdiagnosis of BWT is very unlikely because most non-Wilms malignancies and benign renal entities present with unilateral lesions. Furthermore, in the collective experience of NWTS, Societé Internationale D’Oncologie Pédiatrique (SIOP), and German and United Kingdom cancer studies, no patient with bilateral tumors has been found not to have Wilms in either kidney. The second often-quoted rationale is to detect anaplasia. Experience from NWTS-4 has demonstrated that needle biopsy is inaccurate for the detection of anaplasia. This failure rate is not substantially improved through open biopsy whereby the detection rate was 1 in 3 in the same study. Furthermore, in prior NWTSG studies, tumor biopsy was considered local spill, in effect upstaging of the tumor. Local spill was associated with an increased rate of abdominal recurrence in NWTS-4. For these reasons, neither open surgical biopsy nor needle biopsy at initial presentation is recommended for most patients as part of the current protocol.
Overview of Bilateral Wilms Tumor Treatment Strategy
In patients with bilateral tumors, a baseline abdominal computed tomographic (CT) scan or magnetic resonance imaging (MRI) is performed, with follow-up imaging at 6 and 12 weeks, as well as at the end of therapy. Intensive up-front chemotherapy with vincristine, dactinomycin, and doxorubicin is then administered for 6 weeks. Patients are reimaged after 6 weeks of chemotherapy; at this point, those tumors that are amenable to partial nephrectomy are completely excised. The use of MR angiography and venography is frequently helpful if surgery is contemplated. Patients with tumors that are not amenable to renal-sparing surgery undergo an open renal biopsy, followed by 6 more weeks of chemotherapy driven by tumor-specific histology. At 12 weeks tumors are reassessed, and partial nephrectomy is performed if feasible. Radical nephrectomy is performed if partial nephrectomy is still not possible at this point ( Fig. 1 ). Delaying surgical resection is not advisable, as prolonging chemotherapy may not lead to a better response and may even jeopardize outcomes in patients with progressive or nonresponsive disease. Patients with Wilms tumor of high-risk histology, such as those with anaplasia, benefit from early resection and histology-guided chemotherapy.
Nephron-Sparing Surgery for Unilateral Tumors
Nephron-sparing surgery in the setting of unilateral Wilms tumor (UWT) is controversial for several reasons. Previously, reasons for considering partial nephrectomy in these children were the 2% to 3% risk of metachronous contralateral Wilms tumor and the risk of developing renal failure in the future. The incidence of renal failure in patients with UWT is reported to be very low. In 1996, the NWTS reported that children with UWT had only a 0.25% incidence of renal failure. Most of the patients in that study experiencing renal failure had Denys-Drash syndrome (DDS), making them more prone to medical renal disease. Only a very small number of patients in this study were observed for more than 20 years, making it difficult to determine long-term effects of complete unilateral nephrectomy in most patients. Patients with DDS or aniridia and UWT are at increased risk of renal failure due to a mutation in the WT1 gene. In the NWTSG Late Effects Study, patients with DDS had the highest risk of early renal failure, but children with the WAGR syndrome (Wilms tumor, aniridia, genitourinary anomalies, and mental retardation) had somewhat higher rates in later years. Renal-sparing surgery for unilateral lesions can be considered in this cohort of patients.
Most unilateral Wilms tumors will not be amenable to partial nephrectomy at diagnosis, with the occasional exception of tumors found on screening ultrasound for children with Wilms tumor-related syndromes. Therefore, preoperative chemotherapy may be necessary to reduce tumor burden enough to facilitate partial nephrectomy. In 2003, Haeker and colleagues reviewed the results of partial nephrectomy in unilateral Wilms tumor from SIOP 93-01/GPOH. Between 1994 and 2001, 37 partial nephrectomies were performed compared with 770 total nephrectomies for unilateral Wilms tumor. Of these 37 patients, 15 underwent partial nephrectomy prior to chemotherapy; 4 of these 37 patients had tumor recurrence, 2 of whom subsequently died. One patient who died had metastatic disease after a partial nephrectomy; the other had a malignant rhabdoid tumor. The other 2 patients had local recurrence, which was completely resected at reoperation. Overall, there was an 8.1% rate of local recurrence after partial nephrectomy compared with only 3.1% in patients who underwent total nephrectomy. Despite the higher rate of relapse after partial nephrectomy, tumor stage, overall survival, and relapse-free survival were not different between those who underwent partial nephrectomy and those who underwent total nephrectomy. There did appear to be a trend, although not statistically significant, toward poor outcomes in those patients with “high-risk” histology after partial nephrectomy. Also, there was a trend toward improved outcomes in those who underwent partial nephrectomy after chemotherapy. Based on these results, the investigators recommended that partial nephrectomy only be considered after chemotherapy for stage I, low-risk or intermediate-risk patients in whom complete tumor resection is possible and in whom tumor-free margins can be confirmed on intraoperative frozen section. Excellent outcomes after nephron-sparing surgery in patients with stage I tumors were also reported by Zani and colleagues in 2005. In their retrospective series, 10 patients with stage I Wilms tumor had undergone nephron-sparing surgery, and at a mean follow-up of 75.3 months, all patients were alive and event-free.
In an earlier retrospective series of 90 patients with Wilms tumor diagnosed between 1982 and 1992, partial nephrectomy was performed in 7 patients with unilateral lesions. Selection criteria for performing a partial nephrectomy were tumor confined to 1 pole of a functioning kidney, occupying less than a third of that kidney, and absence of invasion into the renal vein or collecting system. Of these 7 patients 2 died of metastatic disease, but no patient had evidence of local recurrence at a median follow-up of 101 months. The kidney remnant appeared to have excellent differential kidney function at follow-up.
Current COG protocols do not allow for partial nephrectomy in cases of unilateral Wilms where a predisposition syndrome does not exist.
Diffuse Hyperplastic Perilobar Nephrogenic Rests
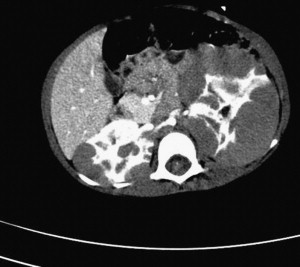
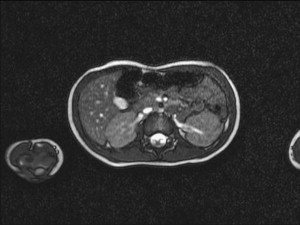
Diffuse hyperplastic perilobar nephrogenic rests (DHPLNR) represent a unique variant of nephrogenic rest (NR) in which the entire cortical surface of the kidney is composed of a hyperplastic NR. These lesions can be quite confusing because they can be very large and appear identical to a Wilms tumor. Imaging with MR or CT scan can be helpful in distinguishing NR from Wilms tumor. NRs appear homogeneous compared with the cortex on contrast-enhanced CT scan ( Fig. 2 ). On MRI, the NR is seen as isointense or slightly hypointense when compared with the cortex on T1-weighted images; the intensity does not change with gadolinium administration. Wilms tumor, on the other hand, has a mixed echogenicity and inhomogeneity. Large rests can respond dramatically to chemotherapy, leaving a rind around the kidney ( Fig. 3 ). When confusion exists, biopsy may be appropriate. Because of the risk of subsequent Wilms tumor development, these patients should undergo a renal-sparing approach ( Fig. 4 ). A discussion of chemotherapy for treatment of DHPLNRs is beyond the scope of this article.
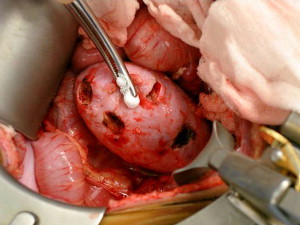
Surgical Technique
In this section the authors describe their preferred technique used for performing partial nephrectomy, as well as a more traditional technique that may be employed at other centers.
At the authors’ institution, surgical planning begins with preoperative MR arteriography and venography. Identifying accessory vessels preoperatively can often facilitate a successful partial resection. One patient at the authors’ institution with bilateral Wilms tumors underwent a left total nephrectomy and right lower pole partial nephrectomy. Fig. 5 demonstrates a right accessory lower pole artery in this patient. Intraoperatively, the lower pole vessels were dissected and ligated, demarcating the parenchyma. This procedure allowed for resection of the lower pole including the tumor and adequate rim of normal parenchyma in a relatively bloodless field. The main renal vessels supplying the upper and midpole of the right kidney did not need to be clamped.
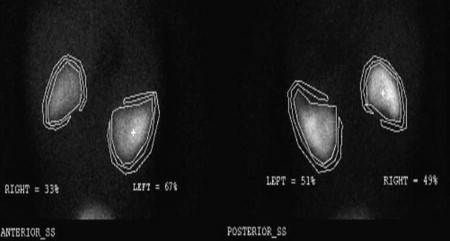
The following is a description of the operative technique. The patient is placed in a supine position after induction of general anesthesia. Wide exposure of the kidney is obtained via a transperitoneal approach. The renal vessels are carefully exposed and encircled with vessel loops and then polar vessels are divided as appropriate. Routine clamping of renal vessels is not performed. Recently, the authors have successfully used the TissueLink device (Salient Surgical Technologies, Portsmouth, NH, USA), a saline-coupled radiofrequency device, to perform simple polar partial nephrectomies and more complex midpolar procedures. Used as a hand device, it works by concentrating sound waves into a conductive fluid, generating heat that seals blood vessels. Proceeding slowly through the renal parenchyma has allowed for a near bloodless field. Given the potential for creating tissue artifact, it is imperative that generous margins be obtained. Intraoperative ultrasound and spinal needle placement have been used to select appropriate planes for dissection, ensuring that the entire lesion is removed with a rim of normal tissue. Pathologic frozen section must be done to ensure that a border of normal tissue is removed. Despite the generation of heat extending into the remaining parenchyma, postoperative radionucleotide imaging has confirmed the preservation of functional parenchyma in the patients after use of TissueLink for partial nephrectomy ( Fig. 6 ). Entry into the collecting system is repaired with 5-0 or 6-0 polydioxanone sutures, and a double-J stent is placed from above. Placement of an omental patch or fibrin sealant is often used to cover the repair and reduce the incidence of urinary leaks. In both cases, postoperative imaging will need to be interpreted with caution, taking into consideration how the repair was performed.
While one can routinely obtain access to the renal vasculature, with modern hemostatic techniques occlusion of the renal artery is not necessary in most cases. Preparation of all patients undergoing partial nephrectomy should include suprahydration, avoidance of unnecessary traction on the renal vasculature, and prevention of periods of hypotension. If renal artery occlusion is required, the administration of 20% mannitol is recommended. For periods of ischemia exceeding 30 minutes, hypothermia is recommended. Lowering renal temperature to 20°C to 25°C decreases cellular activity, providing a protective window of up to 3 hours during renal occlusion. Surface cooling with ice-slush is accomplished by mobilizing the kidney, and then surrounding it with bowel bags into which ice-slush is poured. Full immersion of the kidney to rapidly lower temperature should be maintained for 15 to 20 minutes after occlusion for maximum protective benefit. Thereafter, it is presumed that portions of the kidney may not be fully immersed at certain times. The authors do not prefer infusion of a cooling solution into the renal artery to cool the kidney because this technique risks injury to the vessel.
Another approach may be to position the patient in a lateral position over the kidney rest for dissection of unilateral lesions. A supracostal incision may be best for adolescents, whereas a partial anterior transverse incision could be used in a smaller child. Gerota fascia is opened laterally, and the entire kidney is freed from the surrounding attachments. Perirenal fat around the tumor is left in place. The vascular pedicle is dissected sufficiently to allow the placement of vascular clamps. The renal capsule is then incised. Occlusion of the renal artery with a rubber-shod clamp may be done; occlusion of the renal vein is not necessary if the main renal artery is occluded. The parenchyma is then bluntly dissected away from normal tissue. Arcuate vessels are sharply divided and suture-ligated using 4-0 or 5-0 chromic sutures. An argon beam laser may be used for coagulation if available. The vascular clamp may be released temporarily to identify bleeding vessels. The collecting system should be closed in a running fashion. The kidney is then closed using 3-0 or 4-0 chromic mattress sutures. Gerota fascia is then reapproximated. A frozen-section examination should be performed to assess for negative margins. A closed suction drain is left in place.
Enucleation of very small tumors may be considered for patients with multicentric tumors in a solitary kidney. A layer of normal parenchyma should be included in the resection when possible. Vascular control is usually not needed. The kidney is mobilized as mentioned previously. After the renal capsule is incised sharply, the tumor is bluntly enucleated by following the plane outside the compressed pseudocapsule of tumor. The collecting system should be avoided if possible. The resection bed is fulgurated with an argon beam laser and packed with oxidized cellulose or omentum for hemostasis. Finally, the capsule is closed.
Ex Vivo Surgery and Autotransplantation
Bench surgery or ex vivo partial nephrectomy followed by autotransplantation is an alternative surgical technique that may be considered. As described in 1975 by Lilly and colleagues, ex vivo surgery may permit performance of partial nephrectomy that may not be possible in situ. According to these investigators, extracorporeal kidney surgery allows “careful definitive tumor excision” in a bloodless field. Removing the kidney from the renal fossa provides the opportunity for a proper regional lymph node sampling. A nephrectomy is performed either via a flank incision or transperitoneally and autotransplanted in the iliac fossa or back into the renal fossa. The iliac fossa provides a convenient place for transplantation because of the relative ease of performing the vascular anastomoses. The ureter may be left intact or may be transected and reimplanted into the bladder. Once outside the body, the kidney is flushed with chilled hyperosmolar electrolyte solution to prolong ischemia time. Ischemic injury is a result of depletion of adenosine triphosphate, which is needed for cellular sodium-potassium pump. Energy requirements are significantly decreased in the setting of hypothermia. Successful performance of bench surgery has been reported in small series of patients with bilateral Wilms tumor. Ex vivo perfusion with organ preservation solution may make it difficult for the surgeon and the pathologist to distinguish between normal renal tissue, nephroblastomatosis, and tumor.
Testis-sparing surgery
Introduction
Prepubertal testis tumors are relatively uncommon, accounting for approximately 1% of all pediatric solid tumors, and occur in 0.5 to 2 per 100,000 boys. In contrast to adult testicular neoplasms, most prepubertal testis tumors are benign and have biologic characteristics that make them amenable to local treatment. The abilities to distinguish malignant from benign neoplasms fairly reliably preoperatively and confirm the histopathologic diagnosis with intraoperative frozen pathology have promoted the practice of testis-sparing surgery for select prepubertal testis tumors.
In 1980, the American Academy of Pediatrics Section on Urology established the Prepubertal Testis Tumor Registry. At that time, little was known about the incidence or biologic behavior of prepubertal testis tumors. Treatment recommendations for testicular neoplasms in this age group were drawn largely from adult data. From that registry, it was concluded that most prepubertal testis tumors were yolk sac tumors; other identified tumors included (in decreasing order of incidence) teratoma, unspecified stromal tumors, epidermoid cysts, juvenile granulosa cell tumors, Sertoli cell tumors, Leydig cell tumors, and gonadoblastoma. A more recent multicenter report suggests that the vast majority of prepubertal testis tumors are benign, with yolk sac tumors accounting for only 15% of such tumors. In this report, teratoma was the most prevalent prepubertal testis tumor, representing 48% of tumors in this cohort of patients younger than 12 years with primary testis tumors; overall, 74% of tumors were benign. Several single-institution series confirm the observation that most prepubertal testis tumors are benign. The discrepancy between rates of malignancy in the Prepubertal Testis Tumor Registry and these reports is likely attributable to underreporting of benign lesions to the registry. In addition, the vast majority of prepubertal testis tumors (in contrast to adult testicular neoplasms) have a single rather than mixed histopathology.
The concept of testis-sparing surgery for prepubertal testis tumors emerged in the early 1980s, when it was first applied to primary testicular teratoma and then to excision of benign testicular cysts. Previously, the approach to prepubertal testis tumors was radical orchiectomy. Elegant histopathologic and clinical analysis of prepubertal testicular teratoma demonstrated that these tumors were uniformly unifocal and were not associated with intratubular germ cell neoplasia (carcinoma in situ). The benign nature of most prepubertal testis tumors has expanded application of testis-sparing surgery to several prepubertal testicular neoplasms.
Prepubertal testis tumors in which testis-sparing surgery has been described
Germ Cell Tumors
Epidermoid cyst
Teratoma
Gonadal Stromal Tumors
Sertoli cell tumor
Leydig cell tumor
Juvenile granulosa cell tumor
Cystic Lesions
Simple intratesticular cyst
Tubular ectasia of the rete testis
Miscellaneous
Testicular adrenal rest tumor/adrenogenital syndrome refractory to medical management.
Preoperative Evaluation
The vast majority of boys with prepubertal testis tumors present with a painless scrotal mass. Other presenting signs and symptoms include pain; trauma, hernia or hydrocele, or cryptorchidism, which leads to testicular imaging; gynecomastia or precocious puberty; or incidental discovery. A thorough history and physical examination should be performed. In addition to the presence of a palpable testicular mass, the presence of signs of precocious puberty should raise suspicion for a Leydig cell tumor, a Sertoli cell tumor, or the adrenogenital syndrome. In boys with gynecomastia and a testicular mass, Leydig or Sertoli cell tumor should be considered.
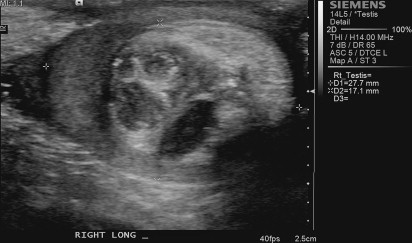
Testicular sonography is routinely performed in patients with suspicion for testicular neoplasm. Scrotal sonography can effectively distinguish testicular from extratesticular lesions and can provide insight as to whether an intratesticular lesion is benign or malignant. Sonographic findings suggestive of a benign lesion include unifocality, anechoic or hypoechoic lesions, distinct demarcation, and hypovascularity. Sonographic demonstration of hypoechoic cystic areas with adjacent hyperechoic areas within a well-circumscribed lesion and surrounding tissue with normal testicular echotexture are suggestive of testicular teratoma ( Fig. 7 ). Color Doppler sonography is likely to demonstrate hypervascularity in testicular malignancies. When considering testis-sparing surgery, it is important to recognize that scrotal sonography often underestimates the volume of residual normal testicular parenchyma. Therefore, the decision to perform testis-sparing surgery should not be based on sonographic estimation of normal testicular parenchymal volume.