Fig. 4.1
Transanal excision of early-stage rectal adenocarcinoma. (a) A single piece of transanal excision specimen with a suture denoting the proximal end. (b) Section shows a well-differentiated adenocarcinoma invades into submucosa arising from a tubular adenoma (Hematoxylin and eosin stain). Evaluation of intact well-oriented section allows assessment of the lateral mucosal and deep margins, which are negative as shown here
Mesorectal Excisions
Patients with rectal cancer who are not candidates for local surgery are treated with a transabdominal resection [5, 7]. In transabdominal resections, TME is recommended. A TME involves an “en bloc” removal of the rectum together with the mesorectum, including associated vascular and lymphatic structures, fatty tissue, and the mesorectal fascia. For lesions in the mid to upper rectum, an anterior resection (AR) extends 4–5 cm below the distal edge of the tumor, followed by creation of a colorectal anastomosis or colostomy. An abdominoperineal resection (APR) is performed when the tumor directly involves the anal sphincter or levator muscles, or when a margin-negative resection of the tumor would result in loss of anal sphincter function and incontinence. An APR involves “en bloc” resection of the rectosigmoid, rectum, and anus, and the surrounding mesentery, mesorectum, and perianal soft tissue [5]. Complete removal of the mesorectum (TME) is important as it contains most of the involved LNs and tumor deposits [7]. In rectal cancer, one of the most important margins is the margin around the mesorectum – circumferential resection margin (CRM). Positive CRM correlates with increased local recurrence rates and decreased survival [8].
It is best to examine the resection specimen in the fresh state as well as following fixation. Surgeons are discouraged from opening the specimen before the pathological gross evaluation unless absolutely necessary, as this may hinder proper assessment of the circumferential resection margins. Prior to opening the specimen, the prosector should identify and differentially ink the serosal and non-peritonealized surfaces and identify the lowest level of peritoneal reflection. It should be emphasized that the entire non-peritonealized surface forms the CRM, which the surgeon has to dissect or cut to detach the bowel from the retroperitoneum. The demarcation of rectum from sigmoid varies and different criteria are applied by anatomist, radiologist, gastroenterologist and pathologist, however, from a oncologic standpoint the tumor location in relation to the peritoneal reflection forms an important landmark. Lower 2/3rd of the rectum lacks any serosal covering, and hence the entire circumferential surface is CRM, while upper 1/3rd or less is partly covered by serosa. During the gross examination one should note the type of operation performed, length of bowel resected, location of tumor with respect to peritoneal reflection, completeness of mesorectal excision, any significant peritoneal pathology (e.g., tumor perforation), tumor configuration, distance of tumor from all resection margins, etc. The quality of surgery of the levator/sphincter area around the anal canal below the mesorectum should also be separately assessed in APR specimens
Accurate assessment of the mesorectum is critical and predicts both local recurrences and distant metastasis. Total mesorectal excision (TME) has been suggested to reduce local tumor recurrence by 10–20 % in various studies [9, 10]. Mesorectal resection can be scored as complete, partially (nearly) complete or incomplete (Fig. 4.2). Complete is defined as intact bulky mesorectum with a smooth surface with only minor irregularities of the mesorectal surface, no surface defects greater than 5 mm in depth, no coning towards the distal margin of the specimen, and smooth appearing CRM on transverse sectioning. Nearly (partially) complete is defined as moderate mesorectal buck, irregularity of the mesorectal surface with defects greater than 5 mm, but none extending to the muscularis propria, and no visibility of the muscularis propria except at the site of insertion of the levator ani muscles. Incomplete is defined as little mesorectal bulk, defects in the mesorectum extending to the muscularis propria, and an irregular appearing circumferential margin after transverse sectioning. Of note, the entire specimen is scored according to the worst involved area.
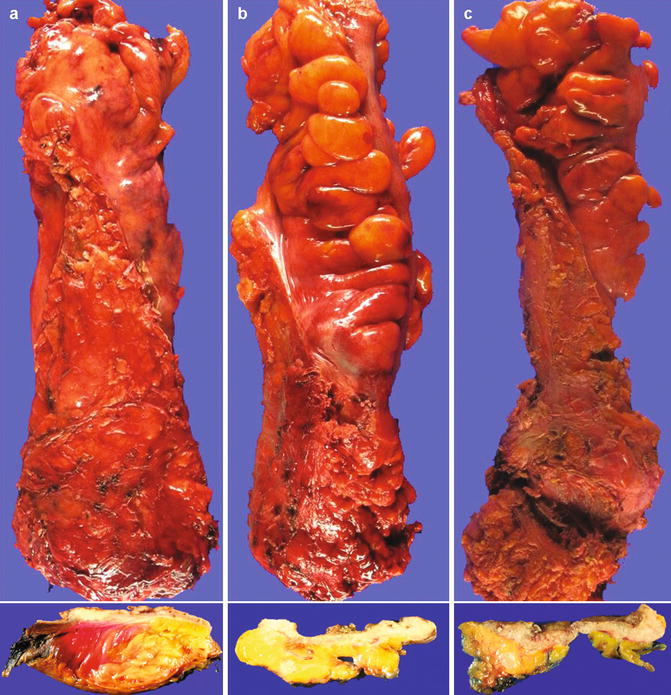

Fig. 4.2
Gross assessment of mesorectal excision in fresh resection specimens as seen in an intact unopened specimen from outside. The inset below each photograph shows a representative cross-section of the formalin-fixed specimen showing the rectal cancer and the mesorectum. (a) Complete mesorectal excision showing smooth mesorectal surface with only minor irregularities; (b) A specimen showing partially (nearly) complete mesorectal excision with irregularity of the mesorectum much deeper than 5 mm; (c) A specimen showing incomplete mesorectal excision showing defects in the mesorectum down to the muscularis propria and >5 mm
The proximal and distal resection margins can be evaluated by either longitudinal sections perpendicular to the margin or en face sections parallel to the margin. The distance from the tumor edge to the closest resection margin(s) should be noted, particularly for low anterior resections. For these cases, a 2 cm long distal resection margin is considered desirable; for T1 and T2 tumors, a 1 cm long margin may be sufficient. Anastomotic recurrences are rare when the distance to the closest resection margin is ≥5 cm [11].
There are no universally accepted guidelines as to how the specimen should be processed, particularly for evaluation of TMEs. Some advocate fixing the specimen by inflation with formalin and then serially slicing the entire specimen transversely, which allows the best way to examine the full circumference of the specimen. However, this process requires longer fixation time, which prolongs the turnaround time. Some suggest that the specimen can be opened similar to large bowel tumors along the border opposite to the tumor, after inking the external surfaces. Following fixation, slices are made at 1 cm intervals and sequentially evaluated. Others make a compromise between these methods by opening both ends along the anterior margin and leaving segment containing the tumor intact. If the tumor segment is longer than 1 or 2 cm, a formalin-soaked gauze or paper can be threaded into the lumen to facilitate fixation. Subsequently the tumor-containing segment can be serially sectioned to yield complete transverse slices. When there is no identifiable tumor especially after neoadjuvant therapy, review of any prior images and palpation the mucosa by inserting a finger into the lumen is helpful. One should carefully open the specimen looking for a scar or shallow ulcer indicating the likely tumor site. For fixation, the opened specimen is ideally pinned to a cork/wax board and immersed in formalin overnight (about 12–24 h).
There is no consensus on the number of sections that should be submitted from the tumor; however, it has been suggested that a minimum of 5 sections are required to detect LVI in most cases [12, 13]. The College of American Pathologist (CAP) also recommends at least 3, and optimally 5 sections should be submitted from the tumor [14]. In general, tumors <3 cm in size should be entirely submitted. For larger tumors, some follow the 1 section/cm rule, merely by convention rather than any evidence based data. If possible, at least one section should also be taken across the direction of the vascular supply close to the tumor to facilitate assessment of venous invasion. At least two sections should be submitted from where the tumor is closet to the peritoneum. Peritoneal involvement may be grossly suspected from areas of serosal pallor, retraction or puckering; however, some cases are only detected microscopically. Conversely, peritumoral fibrosis and inflammation can often simulate peritoneal invasion, and microscopic confirmation is always necessary. If the tumor has an adenomatous edge, appropriate sections should be taken to demonstrate it.
The remaining uninvolved mucosa should be carefully inspected and sections should be submitted from any mucosal bumps, polyps, or abnormalities. Representative random sections should also be taken to ensure there is no unsuspected underlying disease (e.g., inflammatory bowel disease). Although it is traditional to take sections of the proximal and distal resection margins, unless tumor extends close (within 2 cm) to one of the margins, this is of little value. However, poorly differentiated tumors may extend primarily in the submucosa and sometimes spread discontinuously via lymphovascular channels leading to positive margins.
Lymph Node Dissection
A careful search for lymph nodes (LNs) forms an important and sometimes the most painful and time-consuming aspect of handling rectal cancer specimens. The number of retrieved LNs appears to vary with age and gender of the patient, tumor grade, tumor site, specimen type, prior therapy and immune status of the patient. Lymph node size is a poor guide to the presence of metastasis in CRC, with metastases often found in small LNs (<5 mm in diameter), hence a diligent search for LNs is required. All grossly negative or equivocal LNs should be submitted for histological examination. Grossly positive LNs may be partially submitted for microscopic confirmation of the metastasis. Most LNs are found in posterior and lateral quadrants of the mesorectum at the level of the tumor and immediately above, less commonly in the anterior mesorectum [15]. There is no universal agreement on the minimum number of required LNs. The minimum number to accurately stage nodal status and predict patient survival with stage II rectal cancer varies from 10 to 23 LNs amongst studies [16–18]. The 7th edition AJCC staging manual and the CAP recommend evaluating 10–14 LNs in CRC resections in patients without neoadjuvant therapy [11, 19], while examination of at least 12 LNs has been proposed by the National Comprehensive Cancer Network (NCCN) clinical practice guidelines for rectal cancer and endorsed by the National Quality Forum and the Commission on Cancer [5, 20]. However, by no means do these guidelines imply that pathologists should stop searching for LNs once 12 have been identified. If fewer nodes are found, reexamining the specimen for additional LNs should be considered with or without visual enhancement techniques [11]. Visual enhancement techniques such as fat clearing solutions [21], methylene blue-assisted LN dissection [22], and acetone elution with subsequent compression of adipose tissue (“acetone compression”) [23] result in dramatically increased LN counts compared to conventional dissection. However, data are insufficient to recommend routine use of these ancillary techniques and practices vary markedly across labs, even within the same region [8, 11].
Use of neoadjuvant therapy in rectal cancer often leads to atrophy of the lymphoid tissue and reduces LN yield. The mean number of LNs retrieved from rectal cancers treated with neoadjuvant therapy is significantly less than that from those treated by surgery alone [24]. When 12 LNs were considered sufficient for staging, only 20 % of rectal cancers treated with neoadjuvant therapy had adequate LN sampling in stage II tumors in one study [24]. To date, the number of LNs needed to accurately stage neoadjuvant-treated cases is unknown, though a minimum of 12 LNs is still recommended. Studies show the number of retrieved LNs is affected by degree of treatment response and the number of LNs should not be used as a surrogate for adequacy of oncologic resection after neoadjuvant therapy for rectal cancer [25, 26]. Visual enhancement techniques facilitate the detection of LNs, however, their utility in the setting of neoadjuvant therapy remains unclear [5, 23]. Nonetheless, great care must be taken to retrieve LNs in any setting for optimal staging and prognostication.
Histological Features of Rectal Cancer and Their Prognostic/Predictive Significance
Histologic Types
Most primary rectal cancers are adenocarcinomas, of which most are conventional gland-forming tumors (Fig. 4.3a, b). However, some special types need to be addressed as they exhibit different behavior and/or molecular phenotype. The special histopathologic sub-types of CRC recognized over the years include (a) mucinous adenocarcinoma: more than 50 % of the lesion is composed of pools of extracellular mucin (Fig. 4.3c); (b) signet-ring cell carcinoma: more than 50 % of tumor cells with prominent intracytoplasmic mucin, typically with displacement and molding of the nucleus (Fig. 4.3d); (c) medullary carcinoma: sheets of malignant cells with vesicular nuclei, prominent nucleoli and abundant cytoplasm exhibiting prominent infiltration by intraepithelial lymphocytes (Fig. 4.3e); (d) adenosquamous carcinoma: tumors show features of both squamous cell carcinoma and adenocarcinoma, either as separate areas within the tumor or admixed; (e) spindle cell carcinoma: a biphasic carcinoma with a spindle-cell sarcomatoid component in which the tumor cells are at least focally immunoreactive for keratins; and (f) undifferentiated carcinoma: tumors lack morphological, immunohistochemical, and molecular biological evidence of differentiation beyond that of an epithelial tumor and have variable histological features [29]. Micropapillary, serrated and cribriform comedo-type adenocarcinomas have been introduced as new distinct histological subtypes of CRC in the new WHO classification [29]. Micropapillary adenocarcinoma shows small clusters of tumor cells within retracted stromal spaces mimicking vascular channels (Fig. 4.3f). Micropapillary adenocarcinoma can be present in combination with other types in variable amounts. Although it is unclear how much of this component is significant, recognition of any micropapillary component, should be reported, as it imparts a significantly worse prognosis and a high incidence of LN metastasis [30]. Serrated adenocarcinoma shows architecture similar to serrated polyps and is believed to arise via serrated pathway of CRC (Fig. 4.3g). The tumors may show MSI-low, MSI-high or have K-RAS or B-RAF mutations, amongst other distinct molecular changes [31, 32]. Cribriform comedo-type adenocarcinoma shows extensive large cribriform glands with central necrosis analogous to breast adenocarcinomas (Fig. 4.3h), and is usually microsatellite stable with CpG island hypermethylation [33].
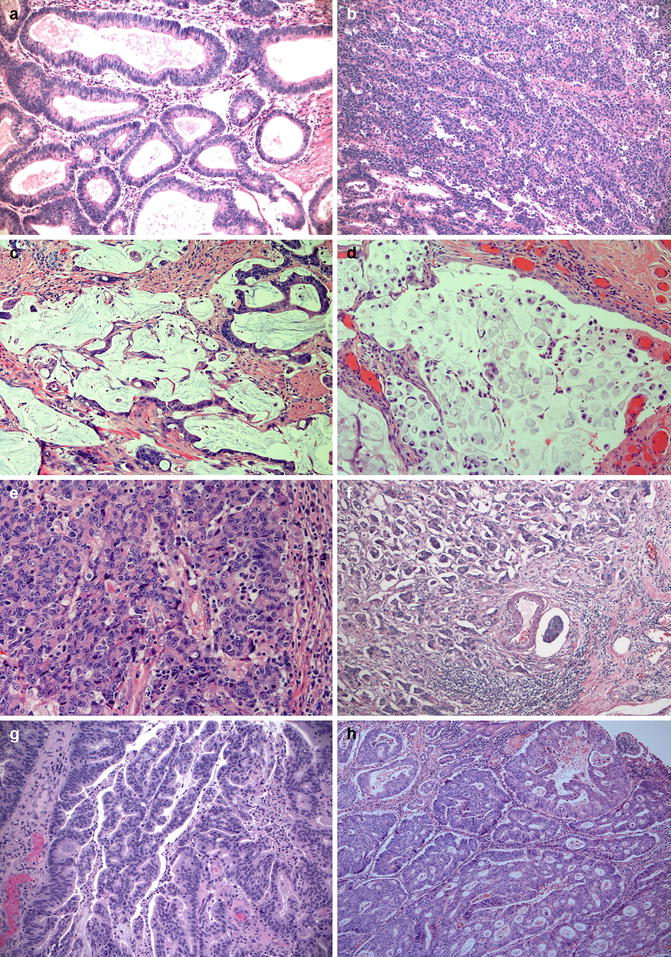

Fig. 4.3
Histologic types of rectal carcinomas. (a) Conventional well-differentiated (Low-grade) adenocarcinoma showing well-formed glands (glandular structures in >95 % of the tumor); (b) Conventional poorly-differentiated (High-grade) adenocarcinoma (glands around 5–50 % of the tumor); (c) Mucinous adenocarcinoma showing large amount of extracellular mucin and tumor cells that often surround these mucin pool and are often low grade; (d) Signet-ring cell carcinoma showing poorly cohesive tumor cells containing single, large mucin vacuoles in their cytoplasm; (e) Medullary carcinoma showing solid sheets of tumor cells with many tumor infiltrating lymphocytes; (f) Micropapillary adenocarcinoma showing small clusters of tumor cells within stromal spaces mimicking vascular channels; (g) Serrated adenocarcinoma showing small papillary epithelial tufts (serrated/corkscrew glandular features); (h) Cribriform comedo-type adenocarcinoma showing glands with a comedo-like pattern and bridging of cells across the lumens filling with necrotic debris. Hematoxylin and eosin stain
Other types of primary carcinoma such as clear cell carcinoma, choriocarcinoma-like carcinoma, large cell or small cell neuroendocrine carcinoma and hepatoid adenocarcinoma do occur in the rectum, but are uncommon. Squamous cell carcinomas are mostly seen as an extension from an anal primary, however, rarely these can be rectal primaries.
Tumor Grading
Adenocarcinomas are graded predominantly based on the extent of glandular appearance. Despite a significant degree of interobserver variability, histologic grade has repeatedly been shown to be an important stage-independent prognostic parameter. Specifically, it has been demonstrated that high tumor grade is an adverse prognostic factor. While traditionally 3- or 4-tiered grading systems: grade 1 (well-differentiated, lesions exhibit glandular structures in >95 % of the tumor) (Fig. 4.3a); grade 2 (moderately differentiated, adenocarcinoma has 50–95 % glands); grade 3 (poorly differentiated, adenocarcinoma has 5–50 % glands) and grade 4 (undifferentiated, < 5 % of tumor with glandular differentiation) have been used, the WHO classification now divides, these into low-grade (well and moderately differentiated adenocarcinomas) (Fig. 4.3b) and high-grade (poorly differentiated and undifferentiated adenocarcinomas) tumors [29]. Studies using the new 2-tiered grading stratification system suggest it is relatively simple, and more reproducibile, while maintaining its prognostic significance. Now CAP also recommends the 2-tiered grading system for grading CRC [34].
In practice, the weakness of the glandular-based grading system is that the estimation of the degree of gland formation is subjective that leads to significant interobserver variability, and ultimately limits the prognostic significance of the histological grade. Furthermore, grade should be established based upon the least differentiated component in heterogeneous tumors; however, the size of such a component has not been specified in any of the systems used in current practice. A proposal that takes into account the extent of poorly differentiated component, defined as a tumor area with no glandular formation, has been proposed recently [35]. Grade 3 was applied to tumors for which the poorly differentiated component fully occupied the microscopic field of an X40 objective lens. For tumors having a smaller component, cancer clusters composed of at least 5 cells, but not forming glands, were counted in the microscopic field of an X4 objective lens where the clusters were the most common. Tumors with less than 10 clusters were classified as grade 1 and those with more than 10 clusters were classified as grade 2. Grade 1 tumors demonstrated 99.3 % cancer-related 5-year survival; grade 2, 86.0 %; and grade 3, 68.9 %, independent of pT and pN stage [35]. Additional studies demonstrated that this tumor grading system based on counting poorly differentiated cell clusters provided significant prognostic information with regards to progression-free survival and was more reproducible than the conventional grading system [36].
Invasive Growth Pattern and Lymphocytic Infiltration
Despite interobserver variability and absence of specific definition and diagnostic criteria, the nature of the advancing tumor margin and degree of lymphocytic infiltrate have been shown to be powerful prognostic indicators in rectal cancer. The majority of rectal cancers show a well- or moderately well-circumscribed (so-called “expanding”) margin. An infiltrative margin, however, is frequently associated with perineural and lymphovascular invasion and confers worse prognosis [37, 38]. As stated above, the recently recognized micropapillary pattern and/or tumor budding, which are often present at the invasive front, are also associated with poor prognosis [39].
Lymphocytic infiltration including tumor-infiltrating lymphocytes (TIL), peritumoral lymphocytes and peritumoral lymphoid aggregates in CRCs has long been considered as an indicator of host immune response to tumor cells, and some studies show a better prognosis in their presence irrespective of the MSI status. Recent studies have shown that lymphocytic infiltration, especially CD3+ T cells or FOXP3+ regulatory T cells along the tumor invasive border or within the tumor stroma, and tumor-infiltrating CD8+ lymphocytes in the cancer cell nests are related to longer survival, early tumor stage, expanding growth pattern and lower levels of LVI in patients with CRC [40, 41]. The peritumoral lymphoid aggregates have been named as Crohn’s-like reaction because it resembles inflammatory response in Crohn’s disease [42]. Recently, TILs and to a lesser extent Crohn’s-like reaction have gained attention because of their association with MSI-H status in most cases [43].
Microsatellite Instability-High Morphology
Identification of microsatellite instability-high (MSI-H) colorectal tumors is important, as DNA mismatch repair deficiency may serve as a prognostic marker of patient outcome, predict response to chemotherapy, and serve as a screening tool for Lynch syndrome. These tumors are commonly located in the right colon, however, they also can be seen in left side with approximately 8 % of rectosigmoid junctional and rectal cancers being MSI-H [44]. MSI-H left-sided and right-sided CRCs [45] have similar histologic features including TILs (Fig. 4.4a), Crohn’s-like lymphocytic reaction (Fig. 4.4b), mucinous/signet-ring differentiation, and/or a medullary growth pattern. These histologic features have a high predictive value for MSI-H; however, a significant portion of patients with Lynch Syndrome or sporadic MSI-H colorectal cancer will be missed if testing for MSI is solely based on tumor morphology or patient’s clinical/family history. Hence, recently universal testing for MSI has been advocated in all newly diagnosed CRCs regardless of patient’s clinical/family history and tumor morphology [46].
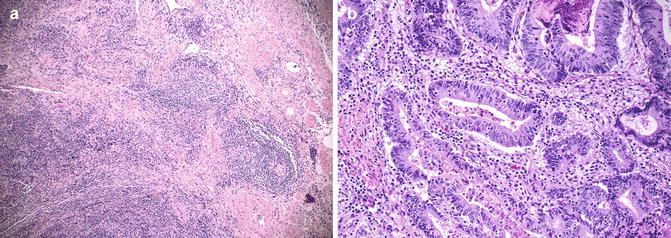

Fig. 4.4
Rectal adenocarcinoma showing with features associated with high microsatellite instability. (a) Adenocarcinoma with Crohn’s-like lymphocytic reaction; (b) Adenocarcinoma with many intratumoral lymphocytic infiltration (Hematoxylin and eosin stain)
Tumor Budding
Tumor budding is described as the presence of detached single cells or clusters of up to 4 or 5 cells (Fig. 4.5a, b) along the invasive tumor front [47]. In contrast to the tumor border configuration (infiltrative or pushing pattern), tumor budding is best identified at high magnification, although one can suspect its presence by the typical cellular myofibroblastic response around the advancing edge of the tumor at low magnification that is typically seen in low grade tumors with pushing borders. This phenomenon has been suggested to represent epithelial–mesenchymal transition, thus increasing the migratory capacity, metalloproteinase expression, and resistance to apoptotic signals, corresponding to a more aggressive biological behavior [48, 49]. Tumor budding scores based on 10 high-power field evaluation show excellent inter-observer agreement and high-grade budding (>10 buds across 10 high-power fields) has been shown to be associated with a higher tumor grade, higher TNM stage, LVI, infiltrating tumor border and reduced survival [50]. Although tumor budding is independently associated with LN and distant metastases, and shorter disease-free and overall survival [51, 52], it is not yet universally reported by pathologists due to the absence of consensus criteria for assessment and cut-off values.
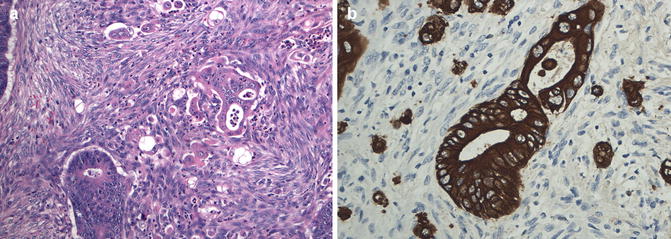

Fig. 4.5
Tumor budding. (a) Infiltrating single tumor cells or clusters of up to 5 tumor cells seen surrounding well formed moderately differentiated glandular structures present at the invasive front of the adenocarcinoma (Hematoxylin and eosin stain); (b) Tumor budding highlighted by cytokeratin immunostain which shows many more tumor cells that are difficult to appreciated on hematoxylin and eosin stain
Lymphatic and Venous Invasion
Vascular invasion is currently an independent prognostic factor in CRC influencing disease progression and survival [14]. The vascular system consists of arterial, venous and lymphatic vessels, however, it is not always possible to distinguish lymphatic channels from capillary-type vessels, because both are small, thin-walled structures. Theoretically, these two types of invasions should lead to different consequences: lymphatic invasion should be predictive of LN metastasis, whereas vascular invasion should be the source of systemic or hepatic metastases. Use of lymphatic endothelial markers, such as podoplanin (D2-40) or lymphatic endothelial hyaluronan receptor (LYVE-1) as well as capillary endothelial markers, such as CD31 or CD34 can distinguish between lymphatic and capillary vessels. However, these are not routinely used in practice. Thus, the presence of small vessel tumor invasion is best reported as lymphovascular invasion (LVI) or angiolymphatic invasion [53]. The AJCC staging manual (7th edition) combined lymphatic and venous invasion into LVI and recommends reporting its presence or absence in CRCs. Large vessel invasion, in particular of extramural venous invasion, has been shown to be an independent powerful indicator of unfavorable outcome and increased risk of synchronous or metachronous distant, especially liver metastasis; however, there are significant interobserver and interinstitutional variations in their recognition due to lack of standard guidelines [53–56].
Perineural Invasion
Perineural invasion (PN) is an often under-reported high-risk pathologic feature in rectal cancer with a widely varying detection rate from 9 to 42 % [57]. It has a similar impact as LVI and should be reported as a prognostic (site-specific) factor, although it does not affect the tumor staging [5, 19]. A 3-tiered grading system (Pn0, no perineural invasion; Pn1a, intramural perineural invasion; Pn1b, extramural perineural invasion) proposed by the Japanese Society for Cancer of the Colon and Rectum (JSCCR) showed 5-year disease-free survival as 88 %, 70 %, and 48 %, respectively, independent of T or N stage [58]. However, most current systems just report the presence or absence of PN.
Tumor Deposits
Tumor deposits (TD) are nodules of tumor present in the pericolonic/perirectal fat that lack recognizable lymphoid tissue. While we have been fixated with the notion that these likely represent nodal metastases with complete replacement of the nodal architecture by the tumor, studies suggest that these may also represent venous invasion, perineural invasion, discontinuous spread of tumor or the advancing edge of the tumor, each with different prognostic implication [59]. TDs have been shown to be associated with reduced disease-free and overall survival [60]. The interpretation of TDs which first appeared in the 5th edition AJCC staging system in 1997 has changed many times over the years. In the current 7th edition, TDs are considered as such recognizing their varied nature. Their number should be recorded in the pathologic report, and they are classified as pN1c in the absence of unequivocal LN metastases, regardless of the pT category; however, once nodal metastasis are identified, the final staging is performed as per the nodal counts (pN1-2). Equating them to LN metastasis likely underestimate their prognostic impact; we expect that this will be addressed in subsequent staging schemes [61].
With regards to rectal cancer it should be noted that neoadjuvant therapy may create residual tumor islands separate from the main tumor mass, which when located in perirectal fat are difficult to differentiate from true TDs. Since these islands are often the remains of advanced-stage tumors, their presence indicates that tumor regression has taken place, which can be linked to a better prognosis. Therefore, some advocate omitting TD terminology after neoadjuvant therapy, and simply to consider such residual islands in the ypT3 category [62].
Serosal (Peritoneal) Involvement
Although the rectum is mostly extraperitoneal, the upper third is variably covered by peritoneum; as such, tumors occurring in upper rectum can potentially involve the serosa and be staged as pT4a. Serosal involvement is an independent prognostic factor in rectal cancer predicting a poor prognosis and should not be confused with CRM involvement [63]. Serosal involvement (T4a) implies a higher risk for intraperitoneal tumor spread and necessitates systemic chemotherapy, whereas positive circumferential margin often indicates increased risk of local recurrence and necessitates treatment modalities that can improve local control, including radiotherapy [64]. Visceral peritoneal involvement is often underestimated and can be easily missed without thorough sampling and/or sectioning and requires careful examination. Data suggest that presence of inflammatory reaction, mesothelial hyperplasia, and/or serosal erosion/ulceration when the tumor is present very close to the serosal surface (<1 mm) is sufficient to indicate serosal involvement and one need not wait to see free tumor cells on the serosal surface [11].
The colorectal serosa is formed by a mesothelial layer supported by a basement membrane containing elastic lamina. Use of elastin stain to demonstrate breach of the peritoneal elastic lamina has also been suggested to serve as evidence of possible serosal involvement [65, 66]. However, the lack of demonstrable peritoneal elastic lamina in all CRCs and the inconsistent sensitivity of the elastic staining reagents limit its wide acceptance in routine practice.
Circumferential (Radial) Resection Margin (CRM)
The CRM represents the adventitial soft tissue margin closest to the deepest penetration of tumor and is created surgically by blunt or sharp dissection of the retroperitoneum [11]. Multivariate analysis has suggested that CRM involvement is a critical factor in predicting cancer-specific survival, local recurrence, and distant metastasis in rectal cancer [4, 67, 68]. A positive CRM in rectal cancer increases the risk of recurrence by 3.5-fold and doubles the risk of death from disease [67]. The distance between the closest leading edge of the tumor and the CRM should be measured and recorded in mm in the report/staging form in all rectal carcinomas [19]. A positive CRM is defined as tumor ≤1 mm from the margin, because local recurrence rates are similar from 0 to 1 mm (Fig. 4.6). This assessment includes both tumor within a LN and direct tumor extension. A positive CRM secondary to LN metastasis in some studies has been associated with lower recurrence rates than that by direct extension [9, 69]. Thus, if CRM positivity is based solely on intranodal tumor, it should be stated in the pathologic report.
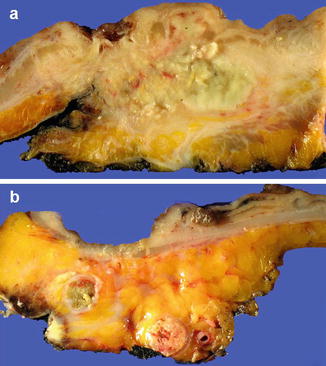

Fig. 4.6
Assessment of circumferential resection margin (CRM) involvement. (a) Slicing through tumor and mesorectum showing tumor within mesorectal fat with negative CRM. This patient had been treated with neoadjuvant therapy. (b) Slicing through tumor and mesorectum showing CRM focally involved by tumor and a tumor deposit present at the CRM
Involvement of the CRM in a patient after therapy with curative intent (e.g., surgical resection for cure) is designated under R classification [19]: R0 for complete tumor resection with all negative margins; R1 for microscopic positive margin and R2 for gross residual tumor. As mentioned above, grading of the completeness of the TME based on gross examination, however, is not assigned any category in the AJCC TNM system and the practice in various pathology laboratories is inconsistent. Of note, the CAP has now omitted the documentation of R category in pathologic reporting of CRCs.
Neoadjuvant Therapy Effect
Multimodality therapy has been successfully implemented in the treatment of locally advanced rectal cancers and increasing numbers of patients now receive pre-operative neoadjuvant therapy. The extent of tumor response to the neoadjuvant therapy has the strongest prognostic impact in the treated rectal cancers. The 7th edition AJCC Cancer Staging Manual [19], the CAP [11] and the NCCN rectal cancer guidelines [5] require comment on neoadjuvant treatment effect that should be reported as: 0 (complete response) – no viable cancer cells; 1 (moderate response) – single cells or small groups of cancer cells; 2 (minimal response) – residual cancer outgrown by fibrosis and finally 3 (poor response) – extensive residual cancer or minimal or no tumor kill. Of note, tumor regression should be assessed only in the primary tumor, LN metastases should not be included in the assessment.
Some tumors show little or no response to neoadjuvant therapy, however, microscopically a variety of morphological changes are often seen after neoadjuvant therapy and need to be recognized (Fig. 4.7a–d). The residual cells may show therapy induced nuclear pleomorphism, increased cytoplasmic eosinophilia and vacuoles, and degenerative changes. Some cases may show presence of neuroendocrine cells, admixed with or without an adenocarcinoma component, and it is speculated that these cells are chemoresistant and hence survive. Significance of residual endocrine cells post chemoradiation has been studied only in few studies which suggest they have no added adverse prognostic impact beyond that dictated by stage of residual tumor [70]. Sometimes mucin pools with or without associated tumor cells are seen. While mucin pools associated with viable appearing tumor cells are staged as per the deepest invasion of the tumor or mucin, evidence suggests that acellular mucin pools present in the bowel wall or LNs behave similar to when no residual tumor is identified [71]. Therefore, acellular mucin pools in specimens from patient receiving neoadjuvant therapy are considered as complete eradication of tumor. A variety of other secondary changes are seen that include stromal fibrosis, inflammatory cell infiltration, calcification and foreign body giant cell reaction which by themselves have no prognostic implication.
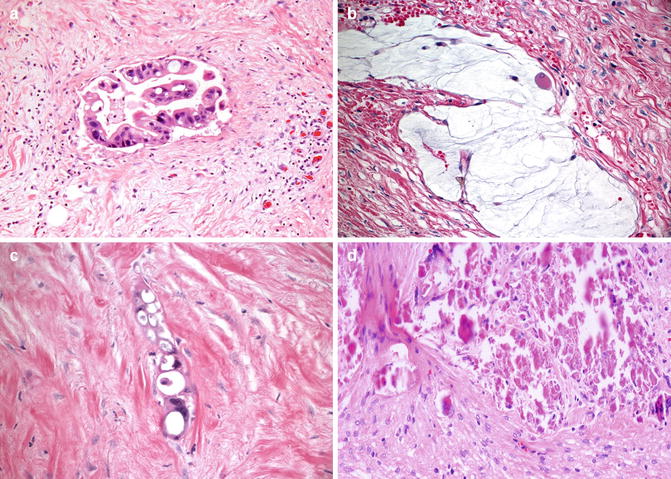

Fig. 4.7a–d
Common morphologic patterns of rectal carcinoma with neoadjuvent therapy effect. (a) Cytoplasmic eosinophilia and vacuoles in the tumor cells; (b) accellular mucin pool; (c) Stromal fibrosis with residual tumors cells showing coarse cytoplasmic vacuoles and hyperchomatic nuclei; (d) Tumor necrosis and stromal fibrosis (Hematoxylin and eosin stain)
The Staging of Rectal Cancer
Dukes Staging System
Pathologic tumor staging remains the fundamental guide for prognostication and treatment decision in managing rectal cancer. Significant improvements have been made in the staging system since the classical proposal introduced by Dukes in 1932 [72]. The original 1932 Dukes classification was based on the extent of disease, as evaluated by the degree of tumor infiltration through the bowel wall, and the presence or absence of LN involvement [72]. Dukes’ A, B, C staging system underwent several subsequent revisions and modifications by Dukes himself as well as other investigators, and it used to be the most popular CRC staging system (Table 4.1). Although Dukes’ staging was a simple, reproducible and widely recognized staging system, it did not take into account the extent of LN involvement, tumor grade, and other pathologic features of tumors. Also there was lack of incorporation of clinical information as well as difficulty in comparing one clinical trial to another due to various versions of Dukes’ classification. The Dukes’ staging system has largely been replaced by the more detailed AJCC/International Union Against Cancer (UICC) Tumor-Node-Metastasis (TNM) system.
Table 4.1
Dukes staging of colorectal cancer
Stage | Description |
|---|---|
A | Growth of primary tumour does not penetrate beyond muscularis propria; no nodal metastases |
B | Growth of primary tumour extends beyond muscularis propria; no nodal metastases |
C1 | Lymph node metastases present but apical node(s) free of tumour |
C2 | Metastases within apical lymoh node(s) |
TNM Staging System
The TNM staging system of the AJCC/UICC has gained popularity over the years and is nowadays the standard staging system for cancers including CRCs [73]. The TNM staging system describes the anatomic tumor extent. It is well known that the best estimation of prognosis in rectal cancer is related to the anatomic extent of disease determined by pathologic examination of the resection specimens. The TNM staging system has the ability to separately classify the individual tumor (T), lymph node (N), and metastatic (M) elements and then group them into different stages. Revisions of TNM staging are made periodically in response to newly acquired clinical data and improved understanding of cancer biology and factors affecting prognosis. Despite some criticisms, periodic update is one factor that makes the AJCC/UICC TNM staging system the most clinically useful staging system and accounts for its worldwide use [74].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






