Fig. 7.1
Hepatic anatomy
Under most circumstances, the right hepatic vein is the largest of the three and enters the superior surface of the vena cava on the right superior aspect of the vena cava. In 85 % of cases, the middle and left hepatic veins exit the liver separately but then join before entering the left anterior aspect of the vena cava. The extrahepatic segments of the major hepatic veins are only 1–2 cm in length. Each hepatic vein has a long (8–12 cm) intrahepatic segment with multiple branches and runs within a portal fissure.
The short hepatic veins enter the anterior wall of the vena cava posterior to the liver. They vary in number, averaging 5–7, and range up to 1 cm in diameter [10]. If the right hepatic vein is small, any large (≥5 mm) short hepatic veins should be preserved when possible if a resection is not being performed.
The two most common anomalies of the hepatic artery are a replaced right hepatic artery from the superior mesenteric artery which courses posterior to the portal vein (15 %) and replaced left hepatic artery from the left gastric artery (12 %). This artery should be carefully sought, as it can easily be missed and cut. When present, this artery courses under the left lateral segment through the gastrohepatic omentum and always crosses posterior to a vagus nerve branch.
Although highly variable, most anomalies of the bile ducts are intrahepatic and do not normally affect the surgery of the liver. One of the most common surgical anomalies of the biliary system is the right posterior duct which crosses over and enters the left ductal system. When this occurs, the insertion is generally just superior to the main biliary bifurcation. This becomes important when performing a left hepatic lobectomy requiring division of the bile duct close to the bifurcation. This can be detected by pre-parenchymal division with a cholangiogram taken through the cystic duct. When this iatrogenic injury goes unrecognized, the patient most often develops a significant bile leak postoperatively.
The fissures on the visceral surface of the liver are important landmarks during liver resection. The important fissures run in what can be presented as a “capital H” configuration (Fig. 7.2) [7, 8]. The right limb of the H runs from the gallbladder fossa posteriorly to the inferior vena cava (demarcating separation of right and left lobes). The left limb of the H runs from round ligament to ligamentum venosum, demarcating dividing line between segment IV and segments II and III. The cross of the H is the porta hepatis.
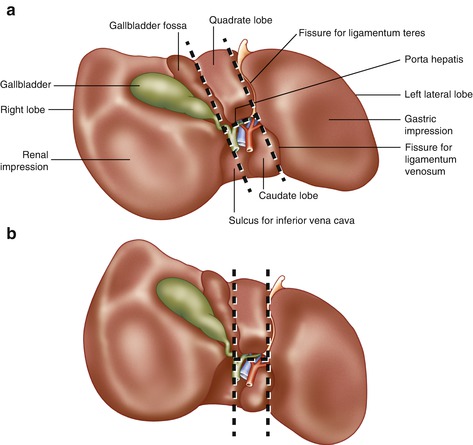

Fig. 7.2
Porta hepatis and features of the visceral surface of the liver. (a) Typical orientation of the H configuration of the portal structures. (b) Incorrect but common depiction of relationship of H configurations parallel with the midsagittal plane of the body
Approach to the Liver
The caudate lobe (segment 1) from the patient’s left provides access to all structures of the liver; it often encircles the inferior vena cava posteriorly [11]. Access is gained to the caudate lobe by elevating the left lateral segment and then opening the gastrohepatic omentum. Avoid an aberrant left hepatic artery from the left gastric artery (see above). The left triangular ligament can be taken down quickly. The left triangular ligament can then be divided with the electrocautery or scissors to the insertion of the left phrenic vein into the left hepatic vein. Divide the falciform ligament down to the hepatic veins, which are more posteriorly than most surgeons believe.
It may also be advantageous to elevate the right side of the liver (often not necessary in urgent situations). To do this, the right side of the liver should be elevated medially and superiorly exposing the right triangular ligament, cut with electrocautery or scissors. Do not drift into the liver parenchyma as the ligament is divided; superiorly, avoid entering the diaphragm. Detach the right adrenal gland from the underside of the liver. Full mobilization of the right lobe of the liver continues with the assistant retracting the right lobe of the liver to the patient’s left; the short hepatic veins are each clipped or tied and divided proceeding from caudal to cephalad until the right hepatic vein is reached. The dorsal ligament must be divided to fully free the liver from the IVC.
Key principles dealing with major hepatic injury in the operating room include the following:
1.
The best help possible with you at the operating table.
2.
Adequate exposure. A trauma laparotomy will start with a midline incision. A right subcostal extension is often necessary to expose retrohepatic venous injury or posterior right lobe injury. On rare occasion, a median sternotomy will assist in exposure of suprahepatic injury to the IVC. Thoracotomy adds little to operative exposure of the liver.
3.
Exposure is achieved with a self-retaining retractor system (many good options available). Retraction in both cephalad and anterior directions is critical; attempt to lift the ribcage off the table with the retractor.
4.
Intravenous access above the diaphragm. Low central venous pressure is maintained throughout the case to minimize bleeding—central venous pressure less than 5 mmHg.
5.
Pedicle ligation demarcates the liver segment to be resected and controls inflow prior to parenchymal dissection.
6.
Minimize blood loss, as excessive blood loss is the major determinant of perioperative outcome. Expertise and technology to minimize blood loss during parenchymal dissection are essential. Operative tools for dissection and coagulation assist in this, such as the tissue link devices, argon beam coagulator, the stapling devices, the LigaSure, and many other advances [12–24]. Many of the instruments available for dissection and coagulation in elective liver resection are not appropriate and essentially ineffective in the patient actively bleeding from a major liver injury.
Stapling Devices
The use of stapling devices has greatly reduced transection time in hepatic surgery and stapling devices are useful in the setting of trauma [25–28]. Crushing staples are best and are fitted with the vascular loads. The capsule of the liver along the intended cutting plane is scored first with the electrocautery, and then the liver parenchyma is crushed and stapled with the stapler.
However, there are a few caveats when using the stapler:
Once the parenchymal transection is begun with the stapler, there will always be bleeding along the staple line (which is best controlled with bimanual compression until the transection is complete). Complete expeditiously.
Place the smaller blade of the stapler into the parenchyma.
The staplers are unaware what they are stapling. What is between the jaws of the stapler will be divided, including the hilar structures and hepatic veins. Thus, the surgeon must be certain of the hepatic anatomy and location of the stapler. In an elective resection, ultrasound can assist with identification of structures. In the trauma setting, a rapid hepatic lobectomy, generally nonanatomic, can be performed with the staplers. Remember the location of the middle hepatic vein running within the main portal fissure. Inadvertent injury to middle hepatic vein will add another major bleeding site. Make certain that the resection line is off-center (Cantlie’s line), toward the lobe being resected, relative to the middle hepatic vein.
The stapler should pass easily into the parenchyma. When it meets resistance, the stapler is either going through the capsule of the liver or into an intrahepatic vascular structure. When this occurs, redirect stapler either deeper or more superficially in the liver. Alternatively, a Kelly clamp can be passed into the liver along the intended transection plane as a guide, before placing the blades of the stapler.
Instruments and Other Techniques
The argon beam coagulator (ABC) is useful for surface injuries to the liver and for subcapsular hematomas. LigaSure or Enseal is useful for division of the liver under controlled circumstances. Salient Surgical Technology devices (formerly TissueLink) are useful tools for dissection of the liver and for hemostasis on the intrahepatic tissue. These devices link radiofrequency with cool saline at the cone-shaped tip of the device. The Cavitron Ultrasonic Surgical Aspirator (CUSA, Tyco Healthcare, Mansfield, MA) combines ultrasonic energy with aspiration. The CUSA dissects tissue, but does not offer coagulation or hemostasis. The Harmonic Scalpel (Ethicon Endo-Surgery, Cincinnati, Ohio) uses ultrasound energy applied to vibrating ultrasonic shears, sealing and dividing vessels up to 3 mm. The bipolar device (the Aquamantys) is useful for hemostasis alone and is quick. However, these devices are not useful for large parenchymal injuries, especially in cracks and crevices that can occur in liver trauma. None of these devices should be used near the main hepatic ducts, as significant thermal injury will occur. If the patient is stable and has undergone any significant resection, the raw liver surface should be treated with one of these devices to minimize the risk of postoperative bleeding or biliary leak.
Thrombin activated factors help seal small bile leaks and stop minor bleeding; they should not be depended upon to stop any significant bleeding.
We do not utilize aortic clamping for isolated hepatic trauma. It affords the surgeon nothing and deprives the other abdominal viscera of inflow.
The Operation
The intrahepatic radical to either lobe may be approached from outside or inside the liver. Formal extrahepatic dissection can be time-consuming, with potential risk because of anatomic variability. Intrahepatic hilar division is as safe as extrahepatic hilar division in terms of intraoperative blood requirements, morbidity, and mortality (Figs. 7.3, 7.4, and 7.5) [15, 16, 29–31]. The stapling devices have facilitated this approach [22, 27]. The intrahepatic approach to the pedicles involves dissection of hepatic parenchyma along the hepatic fissures with control of the pedicles within the liver (see Fig. 7.2). Intraoperative ultrasound is invaluable in identification of the portal triads or hepatic veins within the liver.
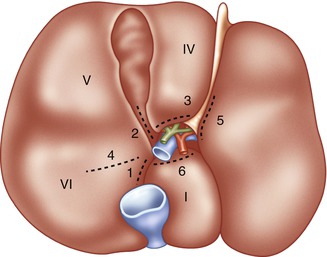
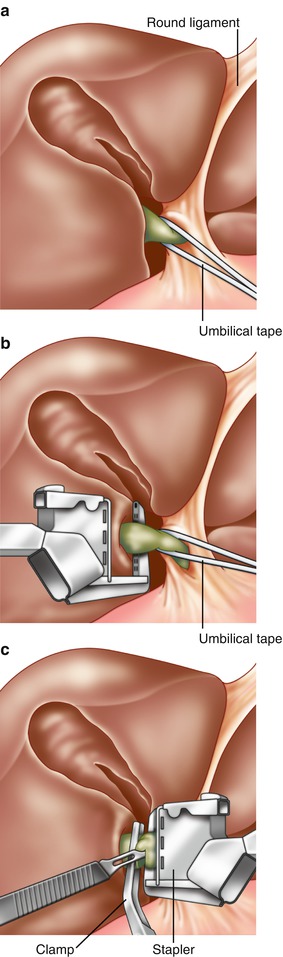
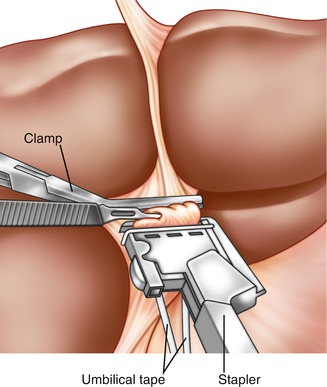

Fig. 7.3
Sites for hepatotomy in portal pedicle isolation. The undersurface of the liver is illustrated. The dotted lines indicate sites for hepatotomy if control of the intrahepatic portal pedicles is desired. Incision at 3 allows lowering of the hilar plate. Incisions at 1 and 2 allow control of the right main pedicle. Incisions at 1 and 4 allow control of the right posterior pedicle. Incisions at 2 and 4 allow control of the right anterior pedicle. Incisions at 3 and 5 allow control of the left pedicle (Reprinted Fong and Blumgart [26], with permission from Elsevier)

Fig. 7.4
Control of the right portal pedicle. After incisions into the liver at sites 1 and 2 in Fig. 7.3, the right portal pedicle is delivered into view. An umbilical tape is placed around the pedicle for control. (b) Placement of the stapler. While constant traction is placed on the umbilical tape to the right, a vascular stapler is applied on the main right pedicle. The traction of the umbilical tape prevents accidental application of staples too close to the hilus. (c) Division of the right portal pedicle. After application of the stapler, a vascular clamp is placed on the specimen side of the portal pedicle. The right portal pedicle is then cut using a scalpel (Reprinted from Fong and Blumgart [26], Copyright 1997, with permission from Elsevier)

Fig. 7.5
Stapler ligation of left portal pedicle. After incisions into the liver at sites 3 and 5 in Fig. 7.3, the left portal pedicle is delivered into view and an umbilical tape is placed around the pedicle for control. While constant traction is placed on the umbilical tape downward, a vascular stapler is applied on the left main pedicle. The traction of the umbilical tape prevents accidental application of staples too close to the takeoff of the caudate branches. After application of the stapler, a vascular clamp is placed on the specimen side of the portal pedicle. The left portal pedicle is then cut using a scalpel (Reprinted from Fong and Blumgart [26], Copyright 1997, with permission from Elsevier)
Large blood loss during parenchymal dissection must be avoided. Dissection through the liver parenchyma to the inflow and outflow vessels without prior vascular control avoids erroneous ligation of portal structures, but bleeding from the transected liver surface may be substantial unless the procedure is performed quickly. The Pringle is often applied as an atraumatic clamp across the hilar structures. However, the authors prefer double-looping a wide vessel loop around the hilar structures, pulling up hard on the vessel loop, and clamping the vessel loop just anterior to the hilar structures. This avoids any potential injury to the common bile duct which may occur even with an atraumatic clamp. The Pringle maneuver or selective inflow occlusion may minimize blood loss, but increases the risk of postoperative liver dysfunction. The normal liver can tolerate up to 120 min of ischemia, as long as done intermittently rather than continuously. Ten to fifteen minutes of clamp time of the portal hepatitis, spelled by 5 min of liver perfusion between clamp times [15, 16, 32].
As mentioned, techniques for liver resection include finger fracture technique, clamp crushing, staplers, ultrasonic dissection, such as the Cavitron Ultrasonic Surgical Aspirator (CUSA, Tyco Healthcare, Mansfield, MA), the water jet dissector, the Harmonic Scalpel (Ethicon Endo-Surgery, Cincinnati, OH), the LigaSure (Valleylab, Tyco Healthcare, Boulder, CO), the TissueLink dissecting sealer (TissueLink Medical Inc, Dover, NH), or radiofrequency-assisted liver transection. The clamp-crushing technique is still widely applied because of its effectiveness and low cost. A recent meta-analysis did not indicate a “benefit of any alternative transection technique on patients’ perioperative outcome compared with the clamp-crushing technique. The clamp-crushing technique remains the reference technique for transection of the parenchyma in elective hepatic resection” [33–35]. For elective resection, we do utilize much of the newer technology, as we will discuss.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








