The incidence of pancreatic cystic neoplasms is rising, in part from detection through the increasing use of high-resolution cross-sectional imaging techniques. Initial diagnosis is generally based on imaging characteristics identified on computed tomography and/or MRI. Endoscopic ultrasound provides further imaging characterization and also enables fluid aspiration and analysis to additionally aid differentiation. The general approach to these lesions includes surgical intervention and/or surveillance imaging. Taking into account diverse presentations, varying malignant potential, and the uncertain natural history of some of these lesions, an evidence-based approach is limited. This article discusses recent updates in the diagnosis and management of cystic neoplasms of the pancreas.
Key points
- •
Cystic neoplasms of the pancreas have diverse presentations, varying malignant potential, and with the uncertain natural history of some of these lesions, an evidence-based approach to management is limited.
- •
There is significant potential for improving the differential diagnosis of cystic neoplasms of the pancreas based on the detection of genetic mutations within cyst fluid.
- •
There are now several guidelines for the management of cystic neoplasms of the pancreas, each with its own limitations.
Introduction
Pancreatic cystic neoplasms were historically considered a rare subset of pancreatic tumors. However, the incidence of these lesions is rising, in part from detection through the increasing use of high-resolution cross-sectional imaging techniques. The reported prevalence of pancreatic cystic lesions on imaging studies ranges from 2% to 16%, and increases with advancing age. These cystic neoplasms of the pancreas are diverse and can be benign or frankly malignant. Given the rising incidence of cystic pancreatic neoplasms and the demonstrated malignant potential of certain subtypes, accurate diagnosis and multidisciplinary management is paramount.
Initial diagnosis of cystic pancreatic lesions is generally based on imaging characteristics identified on computed tomography (CT) and/or MRI. Endoscopic ultrasound (EUS) provides further imaging characterization, often with increased resolution, and also enables fluid aspiration and analysis to additionally aid differentiation. Cyst fluid analysis commonly involves biochemical and cytologic characterization, and in certain cases, assessment for genetic mutations. After diagnosis, the general approach to these lesions includes surgical intervention and/or surveillance imaging. Taking into account diverse presentations, varying malignant potential, and the uncertain natural history of some of these lesions, an evidence-based approach is limited. Consensus guidelines by experts attempt to bridge this gap, and research is ongoing. This article discusses recent updates in the diagnosis and management of cystic neoplasms of the pancreas.
Introduction
Pancreatic cystic neoplasms were historically considered a rare subset of pancreatic tumors. However, the incidence of these lesions is rising, in part from detection through the increasing use of high-resolution cross-sectional imaging techniques. The reported prevalence of pancreatic cystic lesions on imaging studies ranges from 2% to 16%, and increases with advancing age. These cystic neoplasms of the pancreas are diverse and can be benign or frankly malignant. Given the rising incidence of cystic pancreatic neoplasms and the demonstrated malignant potential of certain subtypes, accurate diagnosis and multidisciplinary management is paramount.
Initial diagnosis of cystic pancreatic lesions is generally based on imaging characteristics identified on computed tomography (CT) and/or MRI. Endoscopic ultrasound (EUS) provides further imaging characterization, often with increased resolution, and also enables fluid aspiration and analysis to additionally aid differentiation. Cyst fluid analysis commonly involves biochemical and cytologic characterization, and in certain cases, assessment for genetic mutations. After diagnosis, the general approach to these lesions includes surgical intervention and/or surveillance imaging. Taking into account diverse presentations, varying malignant potential, and the uncertain natural history of some of these lesions, an evidence-based approach is limited. Consensus guidelines by experts attempt to bridge this gap, and research is ongoing. This article discusses recent updates in the diagnosis and management of cystic neoplasms of the pancreas.
Types of pancreatic cystic neoplasms
Serous Cystadenoma
Serous cystadenomas constitute 1% to 2% of exocrine pancreatic tumors with 80% found in women older than 60 years and are therefore sometimes referred to as the “grandmother” tumors. This lesion is considered benign and is typically found incidentally. Occasionally, larger tumors can cause mass effect on surrounding structures, leading to symptoms, such as nausea or abdominal discomfort.
Serous cystadenomas are comprised of multiple cysts usually measuring less than 2 cm in size and separated by thin septations that are lined by epithelial cells ( Figs. 1–3 ). The appearance is often described as a “cluster of grapes.” On cyst fluid analysis, hemosiderin-laden macrophages are seen histologically in 43% of cases. Characteristically, the cyst fluid has low levels of amylase (<250 IU/L), carcinoembryonic antigen (CEA; <5 ng/mL), and serum carbohydrate-associated antigen 19.9 (CA-19.9; <37 U/mL).
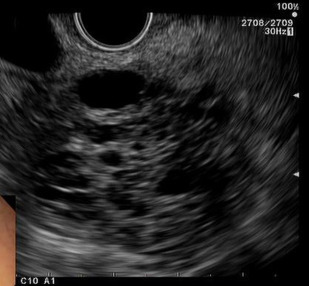
Recent research into serous cystadenomas has focused on their pathogenesis. Clinical case series have suggested an association with von Hippel-Lindau disease, which may implicate a mutation in this gene.
Given the benign nature of the serous cystadenoma, no further follow-up is needed for small cysts once a diagnosis has been made. Larger cysts may demonstrate an increased rate of growth (approximately 2 cm per year), and should be followed with serial imaging to determine need for resection. The interval and length of time surveillance imaging should be conducted remains unknown.
Intraductal Papillary Mucinous Neoplasm
Intraductal papillary mucinous neoplasms (IPMN) are mucin-producing tumors that were first described as a distinct pancreatic neoplasm in 1982. They may arise from the main duct (main-duct IPMN), the side-branches of the main pancreatic duct (side-branch IPMN), or both (combined-type or mixed IPMN). IPMNs demonstrate a variable natural history, from slow-growing, local lesions to invasive and metastatic tumors. There is geographic variation with regard to distribution of IPMN among the sexes. The mean age of diagnosis is approximately 65 years.
Histologically, an IPMN is characterized by the growth of intraductal mucin-producing columnar cells with differing degrees of dysplasia, and supported by pancreatic parenchyma with fibroatrophic changes. These lesions distend the affected pancreatic duct with mucin. In the case of side-branch IPMNs, this gives the appearance of a pleomorphic cystic mass in the pancreas that communicates with the main pancreatic duct ( Fig. 4 ). Identifying this communication is important, because other neoplastic cystic neoplasms generally do not communicate with the pancreatic duct. Main-duct IPMNs are characterized by a focal or diffuse dilation of the main pancreatic duct. Combined duct IPMNs have features of both main-duct and side-branch IPMNs ( Fig. 5 ).
Side-branch IPMNs are commonly found in the uncinate process, but can be seen throughout the pancreas. They may be solitary or multiple. Given the importance of demonstrating connection of the cyst to the main pancreatic duct, magnetic resonance cholangiopancreatography (MRCP) and EUS have emerged as important noninvasive ways of making the diagnosis. Arakawa and colleagues have also demonstrated that findings made on MRCP may correlate with findings at histopathology.
IPMN cyst fluid may stain positive for alcian blue and mucicarmine, highlighting the presence of mucin. IPMNs may have a variable amylase level, reflecting communication with the pancreatic duct, and a high CEA level (>200 ng/mL).
IPMNs are further classified into intestinal, pancreaticobiliary, gastric, and oncocytic-types. This classification is highly predictive of their biologic behavior, but of limited clinical use because preoperative determination of subtype is presently not possible. The main-duct IPMN is primarily of the intestinal-type and is considered highly likely to harbor malignancy. Resection is thus recommended for all patients with main-duct IPMN. However, recent studies have suggested that there may be substantial variation in malignant potential of main-duct IPMNs. In a small study by Abdeljawad and colleagues, absence of symptoms and main duct size less than 8 mm was associated with a lower malignancy risk (25% vs 69%).
The side-branch IPMN is considered less likely to harbor or develop into malignancy and management often includes surveillance after risk stratification (discussed in detail later). Mixed-type IPMN have features of both side-branch and main-duct IPMN. These are thought to have the same malignant potential as main-duct IPMN. A study by Sahora and colleagues suggested that a subset of mixed-type IPMN, which they termed minimal mixed-type IPMN (defined as absence of gross abnormalities except for dilatation of main pancreatic duct and noncircumferential microscopic involvement of main pancreatic duct), may be biologically similar to side-branch IPMN both with regard to demonstrating gastric-type epithelium and low risk of malignant transformation.
There is ongoing research into additional noninvasive markers of high-risk IPMNs. Recently, researchers have identified serum N-glycan as a potential biomarker. In a study of 79 patients with IPMNs, 13 of which were invasive, high levels of fucosylated complex-type glycans, especially m/z 3195, correlated with invasive IPMN. Yabusaki and colleagues have also identified a polymorphism in the vascular endothelial growth factor gene (VEGF) as associated with malignant transformation in IPMNs. Analysis of the secretin-stimulated pancreatic juice collected from the duodenum may also provide insight into patients with IPMNs who have high-grade dysplasia or even pancreatic cancer. Studies, albeit in heterogeneous populations, have identified TP53, GNAS, and KRAS in pancreatic juice as potential candidate genes.
Mucinous Cystic Neoplasm
Mucinous cystic neoplasms (MCN) account for approximately 2.5% of exocrine pancreatic tumors. These occur almost exclusively in women (99.7%) with a mean age of occurrence of 50 (range, 20–82 years). Therefore, these have often been referred to as the “mother” cyst. They are commonly found in the pancreatic body and tail, because of the close proximity of the female gonad to the pancreatic tail during embryologic life. MCN include the benign but potentially premalignant mucinous cystadenoma; borderline MCN; MCN with carcinoma in situ; and the most aggressive form, mucinous cystadenocarcinoma.
MCN are characterized by an encapsulated and dominant round or oval cyst, with average sizes ranging from 6 cm to 11 cm. Histologically, the cyst is comprised of ovarian-type stroma, unique among the cystic neoplasms, with epithelial elements consisting of tall columnar cells with abundant intracellular mucin ( Fig. 6 ).
On CT and MRI studies, enhancement of the capsule along with enhancement of any septations or mural nodules is depicted after administration of contrast material ( Figs. 7 and 8 ). As with IPMN, cyst fluid may stain positive for alcian blue and mucicarmine, highlighting the presence of mucin. On cyst fluid analysis, there is a variable amylase level, typically a high CEA (>800 ng/mL) level and, when malignant, also an elevated CA-19.9 level.
With MCN, there is significant histopathologic variation, with portions of relatively benign-appearing epithelium adjacent to areas of invasive carcinoma. Thus, a biopsy to determine benign versus invasive disease is unreliable. Given the malignant potential of these lesions, and the relatively young age at diagnosis, surgery is traditionally considered for all patients. Although imaging characteristics (solitary, location, presence of capsule, and no communication with the main pancreatic duct) often distinguish a MCN from an IPMN, a study by Nagashio and colleagues suggested that a combination of cyst fluid CEA and CA-125 may also aid in distinguishing these two entities.
Solid Pseudopapillary Neoplasm
The solid pseudopapillary neoplasm (SPN), formerly known as solid and papillary epithelial neoplasm, is a rare pancreatic tumor with less than 1000 cases described in the literature. SPN occurs most commonly in young (mean age, 25 years) women (85%). Recent data have highlighted specific molecular aberrations in the pathogenesis of SPN including CTNNB1 mutations and activation of the Wnt/beta-catenin pathway, distinct from the KRAS, TP53, and SMAD4 mutations typically observed in pancreatic ductal adenocarcinoma.
SPNs are generally benign or low-grade malignant tumors. As with serous cystadenomas, large SPNs can become symptomatic because of mass effect on surrounding structures. On CT, SPNs are generally characterized as well-demarcated, encapsulated, large, cystic and solid masses. In cystic and solid tumors, the solid tissue components are generally noted at the periphery, with central areas of hemorrhage and cystic degeneration noted more centrally. A key diagnostic finding of SPN is the presence of a fibrous capsule that encompasses and surrounds the tumor. Postcontrast administration, the capsule and solid components enhance on CT and MRI. EUS/fine-needle aspiration (FNA) is important in the diagnosis of this lesion, increasing the diagnostic yield to more than 80% and also assisting in risk stratification. In a small retrospective study, focal discontinuity of the capsule on imaging was associated with malignancy. In a retrospective study of 97 patients who underwent resection, positive reactivity to Ki-67 was more common in tumors with malignancy.
SPNs, because of their large size at presentation and possible malignant potential, are generally resected. Occasionally, enucleation has been performed, made possible by the fibrous capsule encompassing the tumor. Overall, patients with this tumor have excellent prognoses with a 5-year survival of 95%. More minimally invasive surgical resection also seems to have similarly excellent outcomes. However, recurrence can occur in almost 10% of patients after 5 years, and thus long-term follow-up is essential. Lymphovascular invasion, synchronous metastases, and local invasion of the tumor capsule are associated with late recurrence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



