The field of pancreas transplantation has evolved from an experimental procedure in the 1980s to become a routine transplant in the modern era. With short- and long-term outcomes continuing to improve and the significant mortality, quality-of-life, and end-organ disease benefits, pancreas transplantation should be offered to more patients. In this article, we review current indications, patient selection, surgical considerations, complications, and outcomes in the modern era of pancreas transplantation.
Key points
- •
The field of pancreas transplantation has evolved from an experimental procedure in the 1980s to become a routine transplant in the modern era.
- •
With outcomes continuing to improve and the significant mortality, quality-of-life and end-organ disease benefits, pancreas transplantation should be offered to more patients.
- •
In this paper, we review current indications, patient selection, surgical considerations, complications, and outcomes in the modern era of pancreas transplantation.
Traditional indications for pancreas transplantation
Type 1 diabetes mellitus (T1DM) is the traditional indication for pancreas transplantation. Caused by autoimmune destruction of the insulin-producing β-cells contained in the endocrine islets of the pancreas, T1DM is a disease of insulin deficiency that ultimately progresses to loss of all physiologic insulin secretion. The prevalence of T1DM in the United States is estimated to be 1,250,000 individuals, and the annual incidence of 35,000 new cases diagnosed is increasing each year. Patients are dependent on intensive insulin therapy delivered by multiple daily injections or continuous subcutaneous insulin infusion pumps for survival. However, owing to the pharmacokinetic and pharmacodynamic limitations of subcutaneous insulin delivery, most patients living today in the United States with T1DM cannot achieve the degree of glycemic control (hemoglobin A 1c < 7%) recommended by the American Diabetes Association. Thus, with the discovery of insulin in 1922, T1DM has changed from a fatal disease to a chronic disease with serious secondary complications resulting from hyperglycemia that manifest many years after disease onset, including retinopathy, nephropathy, neuropathy, and cardiovascular disease.
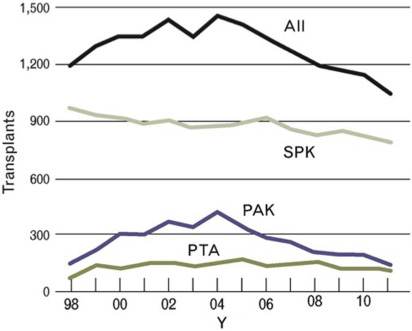
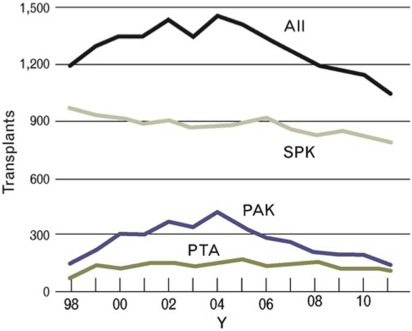
Pancreas transplantation is a proven therapeutic treatment option for adults with insulin-dependent diabetes and is superior to intensive insulin therapy with regard to the efficacy of achieving normoglycemia and control of diabetic secondary complications. Despite improving outcomes for patient and graft survival, the rate of pancreas transplants performed in the United States has steadily decreased since the early 2000s ( Fig. 1 ). The exact reasons for this decline are not fully understood, but potentially include changes in referral patterns, improvements in insulin delivery technologies resulting in delayed progression to advanced diabetic nephropathy, and changes in patient demographics shifting toward more obesity affecting patients with T1DM. A recent decrease in donor organ quality is also likely a contributing factor, because only approximately 15% of US deceased donors in 2013 donated a pancreas for transplantation. This is not a surprising trend, given that the US donor population is becoming increasingly aged, obese, and diabetic, all factors that adversely affect pancreatic graft functional outcomes. Despite these adverse trends, overall short-term technical success rates of pancreas transplantation have improved in recent years.
Traditional indications for pancreas transplantation
Type 1 diabetes mellitus (T1DM) is the traditional indication for pancreas transplantation. Caused by autoimmune destruction of the insulin-producing β-cells contained in the endocrine islets of the pancreas, T1DM is a disease of insulin deficiency that ultimately progresses to loss of all physiologic insulin secretion. The prevalence of T1DM in the United States is estimated to be 1,250,000 individuals, and the annual incidence of 35,000 new cases diagnosed is increasing each year. Patients are dependent on intensive insulin therapy delivered by multiple daily injections or continuous subcutaneous insulin infusion pumps for survival. However, owing to the pharmacokinetic and pharmacodynamic limitations of subcutaneous insulin delivery, most patients living today in the United States with T1DM cannot achieve the degree of glycemic control (hemoglobin A 1c < 7%) recommended by the American Diabetes Association. Thus, with the discovery of insulin in 1922, T1DM has changed from a fatal disease to a chronic disease with serious secondary complications resulting from hyperglycemia that manifest many years after disease onset, including retinopathy, nephropathy, neuropathy, and cardiovascular disease.
Pancreas transplantation is a proven therapeutic treatment option for adults with insulin-dependent diabetes and is superior to intensive insulin therapy with regard to the efficacy of achieving normoglycemia and control of diabetic secondary complications. Despite improving outcomes for patient and graft survival, the rate of pancreas transplants performed in the United States has steadily decreased since the early 2000s ( Fig. 1 ). The exact reasons for this decline are not fully understood, but potentially include changes in referral patterns, improvements in insulin delivery technologies resulting in delayed progression to advanced diabetic nephropathy, and changes in patient demographics shifting toward more obesity affecting patients with T1DM. A recent decrease in donor organ quality is also likely a contributing factor, because only approximately 15% of US deceased donors in 2013 donated a pancreas for transplantation. This is not a surprising trend, given that the US donor population is becoming increasingly aged, obese, and diabetic, all factors that adversely affect pancreatic graft functional outcomes. Despite these adverse trends, overall short-term technical success rates of pancreas transplantation have improved in recent years.
Categories of pancreas transplantation: simultaneous pancreas and kidney transplantation, pancreas after kidney transplantation, and pancreas transplantation alone: broad indications
Simultaneous pancreas and kidney transplantations (SPK) constitute the majority of pancreas transplants performed in the United States, and account for approximately 70% of all pancreas transplants performed (see Fig. 1 ). An SPK, where the pancreas and kidney are recovered from the same donor and transplanted in the same operation to a single recipient, is indicated in adult patients with insulin-dependent diabetes and chronic renal failure of any cause. Although most candidates have kidney disease that is presumed owing to diabetes, in the occasional patient there are other or multifactorial causes. The addition of a pancreas at the time of kidney transplantation can normalize glucose control, providing immediate amelioration from hypoglycemia and metabolic instability and long-term stabilization and sometimes improvement of secondary diabetic complications. The uremic patient with diabetes is an excellent candidate for an SPK transplantation because the immunosuppressive medications that are needed are similar to those for a kidney transplant alone and they are often able to receive a high-quality deceased donor kidney with a shorter waiting time than if they were waiting for a deceased donor kidney transplant alone. As long as the cardiac and surgical risks of the dual operation are considered acceptable, the benefits of adding a pancreas transplant to ameliorate diabetes can extend to prolonging survival of the transplanted kidney that is protected by the pancreas from the development of recurrent diabetic nephropathy.
The second most frequent category in which pancreas transplantation is performed is pancreas after kidney transplantation (PAK). A PAK can be offered to patients with T1DM who have received a previous kidney transplant from either a living or deceased donor or had a prior SPK and suffered loss of the pancreas allograft. This group accounts for approximately 20% of patients undergoing pancreas transplantations in the United States (see Fig. 1 ). An important consideration for this patient population is that of surgical risk and the state of their kidney allograft function, because other risks such as cardiac and immunosuppressive medication-related complications should be low when the patient has already demonstrated absence of cardiac events after the kidney transplant procedure and has tolerated the posttransplant medication regimen.
Pancreas transplantation alone (PTA) is indicated in selected patients with T1DM and normal native kidney function, and makes up the smallest percentage of pancreas transplants performed, roughly 10% (see Fig. 1 ). In this situation the key consideration, in addition to surgical risk, is an assessment of the risk of immunosuppression, and to determine if that risk is outweighed by the risk of diabetes treated with intensive insulin therapy. For these patients, the risk-versus-benefit calculation typically favors PTA in patients who are good surgical candidates with low cardiac risk profiles and who are unaware of impending hypoglycemia owing to loss of counterregulatory mechanisms and hypoglycemia-associated autonomic failure. Various studies have estimated the frequency of hypoglycemia unawareness to be between 15% and 25% of patients. These patients may have frequent and undetectable hypoglycemic episodes that can result in loss of consciousness and life-threatening accidents without warning. More subtly, but just as concerning for some patients, is the fact that frequent unanticipated hypoglycemia can affect employment, familial obligations such as child rearing, and the ability to keep a driver’s license, as well as creating a constant state of fear in the patient and their caregivers. Clinical practice recommendations are available to guide assessment and management for the treatment of T1DM complications by such problematic hypoglycemia, including the consideration of pancreas, or where available outside the United States, isolated islet transplantation. In other patients, a less common indication for PTA is severely labile glycemia that may be associated with frequent emergency room visits for diabetic ketoacidosis despite compliant behavior.
Expanding indications for pancreas transplantation
Historically, pancreas transplantation has generally been considered suitable only for insulin-dependent T1DM patients, classically lean, ketosis prone, unable to produce insulin because of autoimmune β-cell destruction, with C-peptide levels that are extremely low or undetectable. This compares with T2DM patients, classically obese, insulin resistant, with inappropriately “normal” to elevated C-peptide levels. However, this binary understanding of diabetes fails to capture the clinical heterogeneity observed in clinical practice. There are retrospective data that support the application of pancreas transplantation to insulin-dependent patients with T2DM that are carefully selected by body mass index (BMI) and insulin requirement criteria to avoid significant insulin resistance and can become normoglycemic after pancreas transplantation. Because such patients less commonly experience the problematic hypoglycemia and related glycemic lability more often present in long-standing T1DM, non-T1DM account for 8% to 10% of SPK transplants performed in the United States and only 5% and 1% of PAK and PTA transplants are performed in this setting.
Nevertheless, because of the rapidly increasing numbers of uremic T2DM patients, and the perceived concern that T2DM patients would overwhelm the current national transplant system, the United Network for Organ Sharing (UNOS) issued regulations that define eligibility criteria for patients with measurable C-peptide as a surrogate for T2DM. In essence, these regulations impose limits on the number of T2DM patients that will receive SPK transplants by restricting eligibility to those patients with a C-peptide of greater than 2 ng/mL and a BMI of less than 28 kg/m 2 . UNOS policy mandates a 6-month review process with allowance for reduction (or increase) in the BMI eligibility threshold by plus or minus 2 kg/m 2 depending on the makeup and size of the waiting list.
Current data suggest that the outcomes are comparable between non-T1DM recipients and T1DM recipients. Although in some series patient survival after SPK transplantation was worse in non-T1DM recipients compared with T1DM recipients, when adjusting for risk factors such as age, obesity, and African American ethnicity, outcomes were not inferior. In addition, overall patient survival in the T2DM SPK patients was superior to those patients undergoing kidney transplantation alone. Thus, in the appropriately selected T2DM patient, pancreas transplantation can be a valuable treatment option.
Additional indications exist for pancreas transplantation in the setting of benign pancreatic disease. These indications include other forms of diabetes as seen after pancreatectomy, performed either for chronic pancreatitis, pancreatic neoplasms (ie, intraductal papillary mucinous neoplasms), trauma, and various pediatric genetic abnormalities, and separately, in patients with cystic fibrosis who develop both pancreatic exocrine and endocrine insufficiency. In these postpancreatectomy and cystic fibrosis patients, there is the added benefit of restoring exocrine function with an enterically drained pancreas graft.
An additional treatment approach for adult and pediatric patients with chronic pancreatitis, patients with benign pancreatic tumors, and traumatic pancreatic injury is that of total pancreatectomy and islet autotransplantation (TPIAT). TPIAT has been associated with 70% to 80% resolution of pain rates and with significant insulin independence rates. The success of insulin independence often depends on the severity of disease and whether the patient has had prior pancreatic surgery. If referred early before the destruction of native islets, or before attempted surgical drainage procedures, TPIAT can yield sufficient islets to achieve glycemic control with little or no exogenous insulin requirement. In contrast with TPIAT, a subsequent pancreas transplant has the disadvantage of requiring lifelong immunosuppression, but the advantage of not only curing endocrine but also exocrine insufficiency. Both transplant options, if successful, can improve the recipient’s quality of life. Thus, for patients undergoing total pancreatectomy for nonmalignant disease, the standard of care is becoming TPIAT or subsequent pancreas transplantation.
Pretransplant evaluation
Age and Body Mass Index
Many centers are reluctant to perform a pancreas transplant in patients older than 50 years of age and according to the International Pancreas Transplant Registry, only approximately 2% of pancreas transplants are performed in patients older than 60 years of age. Historical data suggested that pancreas transplantation was associated with greater morbidity and mortality when recipients were over 45 years of age. In the context of improved outcomes and the diabetes mellitus population living longer, older patients are now being listed for pancreas transplantation.
Siskind and colleagues published in 2014 a study of age-stratified pancreas transplantation outcomes using the UNOS/Scientific Registry of Transplant Recipients (SRTR) database. The investigators showed, not unexpectedly, that older patients (age 50–59) had shorter patient survival compared with younger patients. However, upon evaluation of death-censored graft survival, the authors observed minimal difference between various age groups. There are few published data on the outcomes of pancreas transplantation in patients greater than 60 years of age, but many of the larger centers are now considering older patients to be potentially eligible as long as the patient has a favorable cardiovascular risk profile and does not have additional comorbidities.
The question of optimal BMI is also a sparsely studied topic. From historical, retrospective, single-center data, obesity has been considered a risk factor for reduced kidney and pancreas graft survival in SPK transplantation for many years. Bedat and colleagues recently addressed the impact of recipient BMI on pancreas transplant outcomes in all 3 pancreas transplant categories using the UNOS/SRTR registry in a comprehensive fashion. The authors demonstrate that (1) overweight (25 kg/m 2 < BMI < 29.9 kg/m 2 ) and obesity (BMI ≥30.0 kg/m 2 ) are associated with a moderate increase in early mortality, (2) overweight and obesity are associated with a moderate increased early pancreas graft loss, (3) obesity, but not overweight, is associated with poorer long-term graft survival, and (4) underweight (BMI <18.5 kg/m 2 ) is associated with poorer long-term patient survival. Although the mechanisms underlying these findings are not understood clearly at this time, recipient BMI is a risk factor for recurrent diabetes developing after pancreas transplantation, and current evidence supports the best glycemic control outcomes with a pretransplant BMI of less than 28 kg/m 2 and prevention of posttransplant weight gain. These are important data to consider in light of the increasing BMI of the diabetic population across the United States, and highlight the critical role for both pretransplant and posttransplant medical nutrition and behavioral modification treatment to achieve and maintain healthy body weight.
Cardiac Evaluation
Cardiovascular disease is the most important comorbidity in patients with diabetes, especially those with diabetic nephropathy. Because of the neuropathy associated with diabetes, patients are often asymptomatic, and the prevalence of significant coronary artery disease in patients with uremic diabetes is estimated to be approximately 40% to 60%. As such, screening studies to detect significant and treatable coronary artery disease is important in the evaluation for pancreas transplant candidacy. In our experience, noninvasive stress test screening misses approximately 50% of pancreas transplant patients who have significant coronary artery disease. Thus, with a high false-negative rate of noninvasive stress tests, high prevalence, and asymptomatic presentation, there is a “perfect storm” for the development of major cardiac events in the perioperative period. Risk factors such as age, duration of dialysis, duration of diabetes, and smoking and family history should be assessed but are not sufficiently predictive, and standard cardiac risk assessment tools have not been validated widely in this population. Hence, we have adopted an aggressive policy of coronary angiography in nearly all dialysis patients and the majority of nonuremic patients. In the preuremic patient, however, this can still present a diagnostic challenge because of the risk of iodinated contrast precipitating dialysis. Fortunately, techniques for coronary angiography have improved significantly and with the avoidance of ventriculograms such that the contrast dye load and consequently the nephrotoxic risk has been reduced considerably. We feel that the benefit of adequately screening these patients outweighs the risk of converting these patients to dialysis dependence and should not serve as an absolute justification to hold off on coronary angiography. Patients with coronary lesions amenable to angioplasty/stenting or bypass grafting should be treated, reevaluated, and then reconsidered for transplantation. The goal of revascularization is to diminish the perioperative risk of significant myocardial ischemia and cardiac events and to prolong the duration of life after transplantation. Patients who have experienced long waiting periods before pancreas transplantation should have their cardiac status reassessed at regular intervals.
Assessment of Peripheral Vascular Disease
Given the high rate of peripheral vascular disease present in the pancreas transplant population it is important to assess the adequacy of the iliac vessels before transplantation. A nonintravenous contrast computed tomography (CT) scan of the abdomen and pelvis is the best option for assessing target vessels, even at times when the clinical examination shows good femoral pulses. A noncontrast CT scan can easily detect iliac artery calcifications as well as aid in operative planning.
Additionally, diabetic patients are at risk for amputation of the lower extremity. These problems typically begin with a foot ulcer associated with advanced neuropathy and/or tibioperoneal vascular disease. Significant distal vascular disease or amputation of the lower extremities is not, however, an absolute contraindication to transplantation.
Assessment of Insulin Requirements, C-peptide, and Autoimmunity
Daily insulin requirements and serum fasting C-peptide levels are assessed to determine the type of diabetes present, the degree of insulin resistance, and so whether the patient will benefit from pancreas transplantation. Patients with high insulin requirements (eg, >1.0 U/kg per day) and high fasting C-peptide levels (eg, >4.0 ng/mL) probably have significant insulin resistance and may not be rendered insulin independent with a pancreas transplant. As an exception, patients who are on peritoneal dialysis may have large insulin requirements owing to the use of dextrose-containing dialysate; this should be taken into consideration when such patients are assessed for transplantation because their insulin requirement will likely decrease when peritoneal dialysis is discontinued after transplantation.
Few studies have examined criteria for pretransplant insulin requirements and C-peptide, which is challenging owing to varying renal insulin and C-peptide clearance mechanisms in patients with stage 4 and 5 chronic kidney disease undergoing transplant evaluation. In addition to kidney function that affects C-peptide clearance, it is important to interpret the C-peptide level with consideration of a concomitantly measured serum glucose that affects C-peptide secretion, because the level may be suppressed in insulin-treated patients experiencing hypoglycemia at the time of collection. The UNOS pancreas allocation system dictates that candidates will have to meet the following criteria for pancreas listing: on insulin and C-peptide 2 ng/mL or less (presumably T1DM) or on insulin and C-peptide greater than 2 ng/mL and BMI less than 28 to 30 kg/m 2 (presumably T2DM).
Finally, for patients with T1DM, pretransplant assessment of autoimmune markers (eg, antibodies against glutamic acid decarboxylase, insulinoma-associated antigen-2, zinc transporter-8, and insulin) should be considered to establish a baseline before possible pancreas transplantation. After transplantation, a new or increasing titer of a T1DM-specific autoantibody may indicate recurrent autoimmunity as a cause for pancreas graft dysfunction and aid in the evaluation of any new-onset hyperglycemia.
Assessment of Problematic Hypoglycemia
Complications of long-standing T1DM, including the development of defective glucose counterregulation and hypoglycemic unawareness, related excessive glycemic lability, and frequent and severe hypoglycemia episodes, may increase the urgency for pancreas transplantation. The most common tool used to assess hypoglycemia awareness is the Clarke Hypoglycemia Symptom Questionnaire. The Clarke method comprises 8 questions characterizing the patient’s exposure to episodes of moderate and severe hypoglycemia. It also examines the glycemic threshold for, and symptomatic responses to, hypoglycemia. A score of 4 or greater implies impaired awareness of hypoglycemia, and should be part of the evaluation of all T1DM patients considering pancreas transplantation. Other more complicated, but more quantitative scoring systems include the Lability Index and the HYPO Score.
Kidney Function
UNOS requires SPK candidates to meet criteria for kidney transplant listing (estimate glomerular filtration rate [eGFR]≤ 20 mL/min or dialysis dependence). For both PAK and PTA candidates, the adequacy of kidney function should be assessed. Unfortunately, some patients being evaluated for PTA may have renal function that is too marginal for PTA but also not advanced enough to be eligible for SPK. In these circumstances, where a PTA candidate has marginal renal function, a trial of tacrolimus therapy can be used to predict the effect of calcineurin inhibitor therapy on postoperative native kidney function. If native kidney functional reserve is deemed insufficient, then the patient is best served by waiting for kidney function to further deteriorate to an eGFR of 20 mL/min or less. The precise eGFR threshold for eligibility for PTA has not been determined, but many experienced centers recommend PTA candidates have an eGFR of greater than 70 to 80 mL/min and allow for microalbuminuria but not macroalbuminuria. The goal is to avoid PTA in patients with vulnerable kidney function owing to early underlying diabetic nephropathy. Because PAK patients are already on CNIs, the threshold for satisfactory kidney function is much lower and patients can be successfully transplanted with a PAK with an eGFR of approximately 40 to 50 mL/min with an expectation of little change in eGFR after pancreas transplantation.
Assessment of Autonomic Neuropathy
Autonomic neuropathy is prevalent in diabetic patients and may manifest as neurogenic bladder dysfunction, gastropathy, and/or orthostatic hypotension. Neurogenic bladder dysfunction is important to consider, especially in patients receiving a bladder-drained pancreas or an SPK transplant. A patient who is unable to empty the bladder completely or who cannot sense a full bladder may predispose the patient to urine reflux and high postvoid residuals. In the long term, these problems may adversely affect renal allograft function, increase the incidence of urinary tract infections, or lead to graft pancreatitis. Impaired gastric emptying is an important consideration with significant implications in the posttransplant period. Patients with severe gastroparesis may have difficulty tolerating oral immunosuppressive medications, predisposing the patient to subtherapeutic levels and graft rejection.
Assessment of Retinopathy
Diabetic retinopathy is a common finding in patients with diabetes and microangiopathy. Although blindness is not an absolute contraindication to transplantation, proper social support should be confirmed to ensure adequate support to help with travel and immunosuppressive medications in the patient with significant vision loss. Annual ophthalmologic examinations are recommended pretransplantation and posttransplantation. Acute normalization of glycemia has been associated with transient worsening of retinopathy, and so stability of retinopathy and provision of any indicated treatment should be ensured before pancreas transplantation.
Screen for Availability of Living Donors
Pursuing a living donor kidney (LDK) transplant before pancreas transplantation (ie, pancreas after LDK) is a viable alternative option for uremic T1DM patients instead of an SPK. The benefits of an LDK versus deceased donor SPK have been discussed in many forums. A reasonable strategy to transplant a kidney and pancreas graft as rapidly as possible in this population is as follows: (i) if the patient does not have an LD, then the best option is an SPK, (ii) if the patient has 1 or more LDs then workup these donors, (iii) if the LDs are HLA identical, proceed with the HLA-identical LDK transplant first followed by a PAK transplant because these kidney grafts have superior long-term survival, (iv) if the LD is a haplotype match or less, then consider an LDK first only if there is a long waiting time for SPK, because receiving an LDK can reduce excess waiting list mortality, and (v) if on the other hand there is a short waiting time for SPK, then it is recommended to proceed with SPK because this option achieves rapid correction of uremia and diabetes with a high-quality kidney and equal short-term and long-term kidney allograft survival to haplotype-matched LDKs. Although initially controversial, recent literature supports increased or equivalent survival in patients who receive a pancreas after LDK versus LDK transplant alone. Additionally, some studies demonstrate improved eGFR in patients after pancreas after LDK versus LDK transplant alone up to 10 years after transplantation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





