Contraindications to R0 Resection
Tumors involving the sciatic nerve
Tumors encasing the common and/or external iliac arteries
Tumors obstructing the bilateral ureters
Tumors causing extensive fixation to the lateral pelvic side wall
Multiple peritoneal metastasis
Metastasis involving the vital structures
Poor performance status
In those patients who are unable to undergo a R0 resection, close monitoring for symptoms that could be palliated by surgical, endoscopic-based, and/or multimodal adjuvant therapies should be performed [4]. Symptomatic patients with stage 4 rectal most commonly present with obstruction or change in bowel habits (20–40 %), bleeding (25–44 %), or pain (6–20 %) [21–23]. Again, it should be stressed that due to the variability in both patients and their tumors, treatment should be individualized and a multidisciplinary team approach is strongly advised.
Operative Intervention
In general, embarking on an operative intervention in a patient with stage 4 rectal cancer in an elective setting is meaningful only if the operative risk is low and the patient has a reasonable life expectancy (at least 2–6 months) [7, 21, 24–29]. In an emergent setting however, the question to be answered is what intervention to offer as an operation may be the only therapy to improve short-term survival [21, 30]. Potentially life-threatening complications include obstruction, perforation and bleeding. The American Society of Colon and Rectal Surgeons (ASCRS) identifies three goals of treatment in the emergent setting: (1) prevent the immediate negative impact of complication such as sepsis and death, (2) achieve tumor control, and (3) allow for the initiation of adjuvant or systemic therapy [4]. Interventions should be individualized based on presenting symptom or symptoms, and available imaging and therapeutic modalities.
Obstruction
Malignant bowel obstructions occur in 10–28 % of rectal cancer patients, and 30–40 % of these resolve with conservative measures such as nasogastric tube (NGT) decompression and bowel rest [7, 21, 23]. These conservative measures are widely-available, inexpensive, and stabilize the patient with low risk of bleeding and perforation from the intervention [31]. However, in patients with persistent or recurrent obstructive symptoms, a more aggressive therapy should be offered. While all operative approaches are palliative in this setting by definition, they can be broadly categorized into venting procedures and resectional procedures.
Venting procedures include gastrostomy tube placement and stoma placement to proximally divert the fecal stream. Venting gastrostomy tube placement, either placed endoscopically or surgically, for bowel decompression allows for discontinuation of an NGT, and potential discharge from the hospital to home or hospice [32, 33]. These should be considered in those patients who have maximized their chemotherapy options, are in the most terminal stages of their disease, or in those who are medically frail from malnutrition and/or other comorbidities. Gastrostomy tube placement is associated with high success rates between 86 and 100 % [34], and immediate improvement in patient symptoms [35]. However, a recent retrospective review demonstrates high major procedural-associated complications (10.2 %), with the majority (59.3 %) of patients being maintained NPO despite percutaneous endoscopic gastrostomy (PEG) placement [36]. Additionally, it should be stressed that in those who undergo PEG tube placement, survival is not significantly improved with median survival of only 66–147 days [34].
A proximal fecal diversion is a great option for those patients who are both fit and willing to undergo an operative procedure. In these patients, the site of obstruction and extent of proximal distension often determines the best surgical option. Diverting fecal stream with a diverting stoma may be the only feasible option in obstructed patients with significant comorbidities or uncertainty of life expectancy, considerable tumor burden or carcinomatosis, or underlying fecal incontinence [1, 37, 38]. In patients with acute malignant obstruction, diverting stoma is the preferred surgical option as it has been demonstrated that patients who undergo emergent primary resection demonstrate worse overall survival when compared to those who underwent elective surgery [39].
While these diverting stomas can be palliative in the sense that they relieve the obstruction, it should always be considered that the stoma itself may have significant adverse effects on the patient’s quality of life with leakage, prolapse or retraction causing limitations in quality of life [40]. One-third of patients develop complications from their stoma including the aforementioned complications as well as skin irritation, pain or partial necrosis [7, 41, 42] not to mention the short-term complications of the procedure itself such as wound infection or ileus [43]. If the decision is made to divert the fecal stream in an obstructed patient a sigmoid or transverse loop colostomy is typically fashioned [1, 44]. Despite placement of diverting stoma, many patients may continue to have pelvic pain due to tumor invasion and/or persistent rectal drainage or bleeding [1]. All of these symptoms worsen the patient’s quality of life.
For these quality of life reasons, resection of the affected segment or a subtotal colectomy with or without reanastomosis (Hartmann procedure) should be considered in those patients presenting with obstruction who are able to tolerate lengthier procedures and who have resectable primary tumors. Historically, obstructions were managed with a three-stage approach in which a diverting colostomy was created during the first operation followed by resection and then reanastomosis during the second and third operations respectively [45]. This was due to patients being very ill with severe dehydration and malnourishment. Additionally, bowel mucosa is friable and patients often do not undergo adequate bowel preparation making reanastomosis undesirable. However, it was found that two-stage procedures were equally effective and associated with shorter hospital stays, and soon a two-stage approach consisting of a Hartmann procedure followed by reanastomosis was adopted. Some centers have now adopted a one-stage approach consisting of a subtotal colectomy with ileocolic anastomosis with equal mortality rates as a two-stage procedure [45].
A low anterior resection (LAR), an abdominoperineal resection (APR), or even pelvic exenteration may be indicated in those with advanced disease and significant symptoms such as severe tenesmus, incontinence, constipation or diarrhea, or intractable pain due to local invasion into nerves [7]. These procedures are less than ideal due to unnecessary risk in terminally ill patients but may be suitable for some patients with longer life expectancies. Tumor size and location in addition to patient factors such as body habitus and comorbidities dictate which procedure is most suitable. In a patient with poor anal function or in a patient who has undergone pelvic irradiation, a Hartmann procedure may be the preferred approach [3]. However, if the tumor involves the sphincter complex, an APR may be the procedure of choice [44]. Additional indications for extensive resection include colovaginal or colovesical fistulae, or tumors that have perforated and are the source of pelvic sepsis [1, 20]. In general, a Hartmann procedure or LAR is preferred over APR for palliation due to decreased perineal wound complications and decreased pain [46].
A laparoscopic approach to diversion or resection may be used as it is associated with a shorter recovery including a faster return of bowel function and shorter hospital stay, and thus a shorter interval to initiating chemotherapy [3]. Additionally, a laparoscopic approach is associated with less pain and fewer postoperative complications making this approach in patients with limited life expectancy appealing [1, 7, 47]. However, a dilated colon may make performing a laparoscopic procedure technically very challenging and not worth the operative risk [1, 3]. Ultimately, the choice in operative approach should dictated by surgeon experience and comfort level.
Perforation
Bowel perforation is a life-threatening complication occurring in 2–9 % of colorectal cancer patients [48, 49]. The patient with stage 4 rectal cancer and bowel perforation is frequently ill and the challenge in this setting is primarily due to the patient’s condition and the emergent nature. Perforation most often occurs near or at the site of the tumor, and occurs as a result of tumor necrosis or adjacent inflammation. Perforation occurring proximal to the site of the tumor is most commonly due to distal obstruction and proximal bowel dilation resulting in local ischemia and transmural necrosis [50].
Prior to pursuing an operative intervention patients and their families should be counseled and expectations of the operation should be managed. It is important in these situations that patients truly understand the implications of undergoing a surgical procedure including both the risks and benefits as well as potential outcomes [51]. The surgical procedure performed will be dependent on the site of perforation and whether intraabdominal sepsis is present. If the perforation occurs proximal to an obstructing tumor, resection of both the affected bowel segment and an oncologic resection should be performed. A perforation at the site of the tumor that is contained should be managed with resection of the involved structures en bloc. Free perforation with peritonitis requires resection of the involved segment and fecal diversion with a stoma [4]. A primary anastomosis may successfully be achieved in the emergency setting but is contraindicated in patients with fecal peritonitis. A staged procedure should be performed in this patient population, especially when in a patient who is requiring vasopressor support or who has major co-morbidities [52]. The goal in performing a staged procedure is to prevent anastomotic dehiscence as this is associated with worse overall survival and need for re-operation [53].
A recent study of 1,004 patients with bowel obstruction in the setting of stage 4 colon cancer was recently undertaken using the Surveillance, Epidemiology and End Results (SEER) – Medicare database. The authors found that median survival after obstruction was less than 3 months with 12.7 % of patients admitted to the hospital dying while inpatient. Moreover, the authors found that the overall ratio of days in the hospital to days out of the hospital did not differ between surgical and nonsurgical therapies (1:5), and surgical intervention was not associated with improved survival [26]. Furthermore, it is clear that patients with bowel perforation in the setting of colorectal cancer demonstrate high morbidity and mortality rates of 43–60 % and 5–40 %, respectively [54–57]. This is thought to be due to a result of both the underlying malignancy and preoperative state of the patient as well as the developing or ongoing sepsis [54]. Given these findings, it is critical that patients have a clear understanding of the risks and benefits of surgical intervention.
Bleeding
Bleeding due to the primary tumor occurs less frequently than obstruction. Patients typically present with chronic blood loss which does not require surgical intervention. However, in patients who present with acute massive blood loss, patients should be aggressively resuscitated and closely monitored in an intensive care setting if unstable [58, 59]. Indications for surgery include persistent hemodynamic instability despite at least six units of blood products, failure of endoscopic techniques to halt bleeding, recurrent bleeding after initial stabilization or accompanied by shock or bleeding requiring greater than three units of blood products per day [60].
Small tumors that cause persistent bleeding and/or symptomatic anemia may be amenable to transanal excision (TAE) or transanal endoscopic microsurgery (TEM) [1, 61, 62]. Transanal procedures have been found to be safe procedures with low morbidity when compared to radical surgery [63–65]. These procedures offer a minimally invasive debulking while minimizing the complications associated with radical surgery, and should be offered to unfit or unwilling patients to more radical procedures [62]. The main limitation to a transanal approach is large obstructing tumors that are unable to be bypassed with a shorter rigid scope [66].
Palliative Resection
Traditionally, palliative resection was, and continues to be performed to prevent obstruction, bleeding and pain in asymptomatic or minimally symptomatic patients. However, it is not currently recommended that patients with asymptomatic tumors undergo routine resection, and systemic chemotherapy should be the primary treatment modality [4, 12]. Despite this, there continues to be a number of proponents for palliative resection in asymptomatic patients with more than two-thirds of patients who present with stage 4 colorectal cancer undergoing resection of the primary tumor according to a study based on the Surveillance, Epidemiology, and End Results (SEER) database [67]. Proposed advantages to palliative resection include improvement in quality of life, prevention of complications of the primary tumor such as obstruction, bleeding or pain as well as improvement in survival rates [8, 68]. Additionally, performing a palliative resection potentially avoids an emergent resection as it has been demonstrated that resection in an elective setting demonstrates a decrease in mortality when compared to resections in an emergent setting [44, 69–71].
Regardless of any proposed benefit, the resection of primary tumors on asymptomatic patients is not without potential risks. Morbidity rates from resections have been demonstrated to be as high as 23–48 % [72], and resections may actually worsen quality of life [30, 73] and lead to poorer survival rates [74]. Additionally, it has been argued that many patients will die from progression of systemic disease rather than the development of a primary tumor complication, and these patients need not undergo an invasive procedure [72, 73, 75]. In fact, a retrospective study by Patel et al. demonstrated that only 4.3 % of patients treated with neoadjuvant chemotherapy progress to complete obstruction and subsequent perforation [43]. In another study of patients undergoing chemotherapy for synchronous colorectal metastasis, only 7 % of patients required intervention for obstruction, perforation or bleeding [76]. Of most concern is that palliative resection delays the initiation of chemotherapy [67, 77]. It is clear that chemotherapy improves both quality of life and survival in patients with unresectable rectal cancer [78, 79]. Thus, interruption or postponement of this therapy to perform resection in an asymptomatic patient is currently not advised.
Despite the risks of resection, a number of retrospective studies have been performed evaluating palliative resection of the primary tumor. Cirocchi et al. recently performed a study for the Cochrane Collaboration evaluating survival in patients undergoing resection of an asymptomatic primary tumor versus chemotherapy. The authors evaluated seven retrospective studies summarized in Table 24.2 [80–86], and found no differences in overall survival and no significant reduction in risk of complication in the resection group [87]. In contrast, Stillwell et al. performed a meta-analysis of eight retrospective studies and found a significant survival benefit in patients undergoing resection of the primary tumor (p < 0.001), and a 7.3 times higher complication rate from the primary tumor in those treated with chemotherapy alone [88]. Recently, the National Surgical Adjuvant Breast and Bowel Project C-10 (NSABP C-10) published the results of their prospective trial evaluating the safety of Bevacizumab to systemic chemotherapy with infusional fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) as the initial treatment for stage 4 colon cancer. The authors found that 87 % of patients did not develop symptoms from the primary tumor over a median follow-up of 20.7 months. They concluded that asymptomatic patients should undergo initial systemic therapy as the rate of serious adverse events from the primary tumor is acceptably low and the majority of patients would avoid unnecessary surgery [89].
Table 24.2
Palliative resection of primary tumor in patients with unresectable colorectal cancer
Study | Study period | Study design | Primary site | Metastatic sites | Resection (n) | Nonresection (n) | Morbidity (%) | Overall survival (months) | Mean follow-up (months) | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
Post resection chemotherapy (n, %) | ||||||||||
Scoggins et al. [80] | 1985–1997 | Retrospective, single institution | Colon and rectum | Liver, lung, omentum, peritoneum | 66 | 23 | 30.3 % Resection | 14.5 Resection | N/A | No survival advantage to resection |
0 (0 %) | 8.7 % Nonresection | 16.6 Nonresection (P = .59) | ||||||||
Tebbutt et al. [81] | 1990–2000 | Retrospective, single institution | Colon and rectum | Peritonal/omental and non-peritoneal/omental | 280 | 82 | 19.3 % Resection | 14 Resection | 30 Resection | Incidence of complications in nonresection group is acceptably low |
0 (0 %) | 23.2 % Nonresection | 8.2 Nonresection (P = .08) | 19 Nonresection | |||||||
Ruo et al. [82] | 1996–1999 | Retrospective, single institution | Colon and rectum | Liver, extrahepatic | 127 | 103 | 20.5 % Resection | 16 Resection | N/A | Significant survival benefit in resected patients |
0 (0 %) | 29 % Nonresection | 9 Nonresection (P < 0.001) | ||||||||
Michel et al. [83] | 1996–1999 | Retrospective, single institution | Colon and rectum | Liver, lung | 31 | 23 | N/A Resection | 21 Resection | N/A | Non-surgical management is rational alternative |
30 (97 %) | 21.7 % Nonresection | 14 Nonresection (P = .718) | ||||||||
Benoist et al. [84] | 1997–2002 | Retrospective, single institution, case control | Colon and rectum | Liver, extrahepatic | 32 | 27 | 19 % Resection | 23 Resection | 24 | Nonresection is option of choice |
14.8 % Nonresection | 22 Nonresection (P = .753) | |||||||||
Galizia et al. [85] | 1995–2005 | Retrospective, single institution | Colon and rectum | Liver | 42 | 23 | 21 % Resection | 15.2 Resection | 21 (5–61) | Benefit to resection |
42 (100 %) | 30.4 % Nonresection | 12.3 Nonresection (P = .003) | ||||||||
Seo et al. [86] | 2001–2008 | Retrospective, single institution | Colon and rectum | Liver, lung, peritoneum, distant node, bone, brain | 144 | 83 | 20.2 % Resection | 22 Resection | 49 (7–89) | First-line chemotherapy is safe without increased risk of intestinal complications |
144 (100 %) | 20.5 % Nonresection | 14 Nonresection (P = .013) |
It has been widely acknowledged that the survival benefit seen in patients who have undergone palliative resection may be due to selection bias [4]. These patients tend to actually be symptomatic and physiologically fitter [30]. Moreover, the studies have all been retrospective reviews evaluating outdated chemotherapy regimens [4]. To address these issues, several prospective studies are currently underway. Although these are studies primarily addressing metastatic colon cancer, guidelines regarding management of late stage rectal cancer may be developed as a result. The University College Hospital in London, England has completed a phase III trial evaluating overall survival in patients with metastatic colorectal cancer receiving chemotherapy with and without surgery. Results have not yet been published [90]. The Dutch Colorectal Cancer Group (DCCG) is currently accruing patients with synchronous unresectable metastatic colorectal cancer to a randomized phase III trial investigating overall survival in patients undergoing resection of the primary tumor followed by systemic therapy compared to patients receiving systemic therapy alone [91]. Additionally, the SYNCHRONOUS trial is a multicenter, randomized controlled trial in Germany evaluating the safety and efficacy of resection of the primary tumor in patients with metastatic colon cancer prior to initiating systemic therapy. Primary endpoint is overall survival with secondary endpoints determining time-to-development of tumor-related complications and intervention required [92].
Endoscopic Interventions
Most patients do not require emergent surgery and obtaining radiographic data becomes important in determining optimal treatment [93]. In addition to localizing the issue, determining the presence of carcinomatosis or ascites is essential as the presence of either is associated with unsuccessful surgery [94]. Nonoperative intervention is the preferred route in those patients with carcinomatosis or ascites, those who demonstrate poor performance status, or those with an unacceptably high operative risk.
Endoscopic-based therapies are an attractive alternative to operative intervention as they are less invasive and can be performed on an outpatient basis without general anesthesia. Endoscopic interventions are able to relieve obstructions, bleeding and/or pain rapidly and effectively. These are important considerations when electing to intervene on patients with limited life expectancy.
Rectal Stents for Obstruction
An alternative to surgical intervention in those presenting with obstruction may be endoscopic stenting to allow patency of the bowel lumen. Given the high morbidity and mortality associated with emergent surgery for colorectal obstruction, self-expandable stents (SEMS) were initially used to convert an emergent surgery to an elective surgery [95]. Placement would allow for rapid decompression and the ability to stabilize the patient with minimal sedation and less cost [96]. This evolved to utilizing SEMS for bridging to definitive surgery or for palliative therapy [97–99].
A SEMS procedure entails placement of a metallic stent across the tumor with the aid of endoscopy, fluoroscopy, or both [100] (Figs. 24.1 and 24.2). Over the course of 24–72 h, the stent expands and becomes incorporated into the tumor by pressure necrosis [96]. Technical success and clinical rates have been demonstrated to be as high as 98.7 and 95.9 % respectively, and 93 and 91 % respectively in the palliative setting [92]. Following stent placement, patients are able to be resuscitated and optimized for any potential subsequent surgical procedure without the need for diverting stoma placement [96, 101–103]. Retrospective studies have demonstrated that stent placement is associated with increased rates of primary anastomosis during subsequent operations and shorter hospital stays [92, 103, 104]. Additional advantages to SEMS include the use of covered stents for colovaginal or colovesical fistulae as well as patients having the ability to undergo chemoradiation with the stent in place [96, 105, 106].
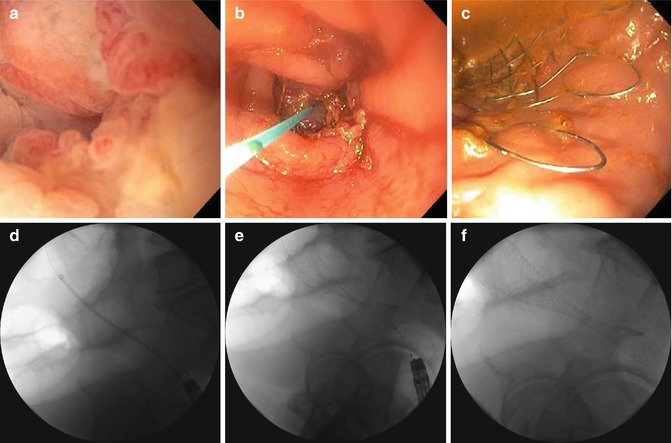
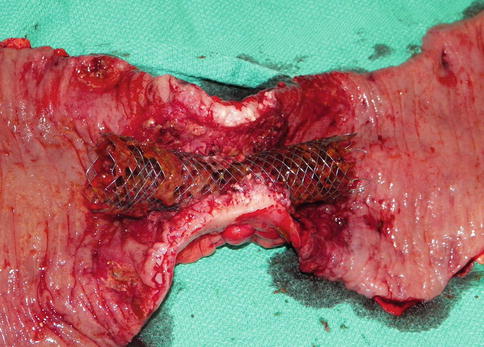

Fig. 24.1
(a) Malignant stricture. (b) Guidewire through stricture. (c) Stent deployment. (d) Guidewire through stricture. (e) Stent deployment. (f) Final stent placement. Placement of a self-expanding metallic stent in an obstructing colon cancer with the aid of endoscopy and fluoroscopy

Fig. 24.2
In situ stent of obstructing colon cancer
Contraindications to the use of colon and rectal stents include perforation and certain characteristics of the tumor that could increase risk of perforation including a long segment of tumor or significant angulation of the colon or rectum [107]. Tumors must be able to be traversed with a guidewire in order for successful stent placement. Additionally, tumors cannot be located within 5 cm of the anal verge to allow for placement of overlapping stents and to prevent the development of pain, tenesmus and incontinence after placement [108]. SEMS is also not indicated in patients with obstruction due to external compression such as metastasis [94].
Although the overall mortality rate of SEMS is low at 1 % [92], complications of SEMS do exist including perforation, migration, reobstruction and bleeding. Perforation rates have been reported to occur in 3.8 % of patients [109]. Although the exact mechanism is unclear, it has been proposed that early perforation is due to balloon predilation, rapid expansion of the balloon or the stent, or guidewire manipulation [109]. Late perforation, occurring less frequently, may be due to friable tissue and poor vascularity particularly in previously irradiated tissue. Additionally, certain chemotherapeutic agents have been associated with an increased risk of perforation [89]. Migration, although typically less serious than perforation, occurs at a rate of 10 % [110]. This is likely due to the tortuosity of bowel and its lack of fixation to adjacent structures and organs in addition to active peristalsis [31, 111, 112]. Tumor shrinkage following chemoradiation, balloon dilation or poorly-sized stents have also been proposed to cause stent migration [110]. Covered stents have been found to have increased rates of migration due to a decrease in tumor ingrowth when compared to the more commonly used uncovered stents [113]. Bleeding occurs in approximately 5 % of patients who have undergone stent placement [109].
A number of retrospective reviews from single institutions have reported primarily positive outcomes. The first randomized trial comparing colostomy to SEMS demonstrated 57 % long-term patency until death [114]. Fiori et al. published another small series of 22 patients with similar morbidity and mortality between colostomy and SEMS groups [115]. A multicenter RCT from the Netherlands was closed prematurely due unacceptably high perforation rate in the SEMS group [116]. A Cochrane Review of five randomized trials evaluating colorectal stents and emergent surgery in malignant colon obstructions, including two of the three previously mentioned, concluded that SEMS has no advantage over emergent surgery. Emergent surgery demonstrates higher clinical success with no differences in overall complication or 30-day mortality rates between the two groups. However, SEMS is safe in this setting with acceptable rates of complications, and the advantage of shorter length of hospital stay [117]. Table 24.3 summarizes several of the studies evaluated in this review [114–116, 118, 119]. Thus, although SEMS has not been demonstrated to be superior to traditional surgical approaches to malignant bowel obstructions, it may be useful in select patients.
Table 24.3
Randomized prospective trials evaluating self-expanding metallic stents (SEMS) compared with surgical intervention
Study | Study period | Site of obstruction | Stent (n) | Surgery (n) | Hospital stay (days) | Morbidity | Primary outcome | Conclusions |
|---|---|---|---|---|---|---|---|---|
Fiori et al. [115] | 2001–2003 | 8 Sigmoid | 11 | 11 transverse colostomy | 2.6 Stent | 0 % Stent | Mean time for GI tract canalization: | SEMS is an effective alternative to surgery |
14 Rectum | 8.1 Surgery (P < 0.0001) | 9.1 % Surgery (P = NS) | 1 day Stent | |||||
3.1 days Surgery (P < 0.0001) | ||||||||
Xinopoulos et al. [114] | 1998–2002 | 12 Sigmoid | 15 | 15 stoma | 28 Stent | 60 % Stent | Efficacy and safety | SEMS is a palliative alternative to colostomy with better quality of life |
18 Rectosigmoid | 14 stent | 60 Surgery (P = N/A) | 13.33 % Surgery (P = N/A) | |||||
van Hooft et al. [116] | 2004–2006 | 5 Descending colon | 11 | 10 | 12 Stent | 72 % Stent | Survival in good health outside of the hospital | Unexpected high rate of perforation (6 of 11) in the stent arm caused early closure of trial |
16 Rectosigmoid | 10 stent | 6 resection with primary anastomosis | 11 Surgery (P = .46) | 10 % Surgery (P < 0001) | ||||
1 did not develop imminent obstruction and was not stented | 1 moved to stent arm due to myocardial infarction | |||||||
Cheung et al. [118] | 2002–2005 | 48 Left-sided colon | 24 | 24 | 13.5 Stent | 8 % Stent | Success of 1-stage operation: | SEMS is a safe and effective bridge to surgery |
11 Hartmann | 14 Surgery (P = .7) | 50 % Surgery (P = N/A) | 67 % Stent | |||||
11 resection with primary anastomosis | 38 % Surgery
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



