Parameter
Unexplained male infertility
Idiopathic male infertility
Semen analysis
Normal
Abnormal
History, physical exam, and endocrine assessment
Normal
Normal
Female factor infertility
Ruled out (no tubal patency, no cervical hostility, or good endometrial receptivity)
Ruled out (no tubal patency, no cervical hostility, or good endometrial receptivity)
Percentage of total cases of male infertility
10–30 %
40–50 %
Contributory causes
1. Sperm dysfunction
2. Auto-antibodies directed against sperm antigens
3. Coital factors interfering with successful fertilization
4. Oxidative stress
1. Age
2. Environmental pollutants
3. Mitochondrial alterations
4. Infective agents (i.e., Chlamydia trachomatis, herpes virus, and adenovirus)
History of harmful toxin exposure
None
None
Infertility Status
Unknown
Known
Categorized under
Male infertility of unknown origin
Male infertility of unknown origin
Although a singular cause of unexplained infertility cannot be identified in many cases, it is of great importance that a systematic infertility workup be performed (Table 10.1). This prevents the oversight of an underlying etiology of UMI [2]. Amidst the modern reproductive technologies of today, this chapter aims to shed light on the concept of UMI, particularly focusing on the diagnosis , treatment, and implicating factors involved in the pathogenesis of this condition.
Assessment of Males with Unexplained Infertility
When a couple seeks assistance for infertility issues, a thorough clinical evaluation of both partners should be performed to rule out identifiable causes and focus the investigation [15, 16]. The initial infertility workup includes a detailed history with an emphasis on the couple’s previous fertility record. This may include a history of recurrent miscarriages and ectopic pregnancies, the period of time in which the patient has been infertile, successful previous pregnancies, and any complications that may have occurred before, during, or after delivery [2, 17]. Furthermore, a thorough coital history from both partners is necessary to reveal any difficulties in regard to intercourse, such as improper sexual technique or inappropriate timing of intercourse [18]. In addition to male infertility evaluation, the female partner should undergo a separate workup, in order to assess the patency of both fallopian tubes , consistency of the cervical mucus, and receptivity of the endometrium to blastocyst implantation [2, 6].
Once a thorough sexual and fertility history has been completed, a comprehensive physical exam should be performed, which can help to exclude anatomical causes of infertility [2). The physician should carefully examine the male patient for any physical aberrations within the structures of the male reproductive system. The penis, in addition to the epididymis, testis, and spermatic cord, should be palpated in order to rule out conditions such as epididymitis, orchitis, and varicocele [19–21]. In particular, all of these conditions promote the aggregation of free radicals or reactive oxygen species (ROS), eventually leading to sperm dysfunction and infertility [19].
Routine Semen Analysis
After the completion of an extensive medical history and physical examination, a routine semen analysis should be the first laboratory test conducted in the infertility workup. This is a cost-effective, non-invasive test that has been integral in the evaluation of male factor infertility for decades [22]. The efficacy of this test lies in the parameters for which it tests, which include pH, volume, color, total count, motility, concentration, and morphology.
Sperm count and motility are the first and most important predictors of fertility potential [23]. Normal values for all semen parameters have been highly associated with improved fertility outcomes and therefore are used for the assessment of infertility [24]. Although semen analysis is the first diagnostic step routinely employed in the evaluation of UMI, it does not identify the exact cause behind the infertility [23, 25].
Therefore, in addition to a comprehensive history, clinical examination, and initial lab assessment, sperm function tests should be performed [26, 27]. Moreover, etiologies related to endocrine or genetic abnormalities should also be explored in the infertile individual [28]. Overall, it is clear that a routine semen analysis cannot be used alone to diagnose infertility. Additional tests are required for the investigation and evaluation of the subfertile male [4].
Upon obtaining a normal semen analysis concurrent with an unremarkable history and physical examination, the physician can make the diagnosis of UMI. At this point, the physician should then explore potential contributing factors implicated in the pathogenesis of the UMI [2, 4]. This includes immunologic factors, genetic integrity defects, fertilization defects, and oxidative stress. The former three are explained in greater detail in subsequent chapters whereas oxidative-induced UMI is the prominent focus of this chapter.
Immunologic Factors
The immune system of the human body naturally protects its cells from the harmful effects of an autoimmune reaction and various pathogens [29]. One such defense mechanism is the blood-testis-barrier, which protects sperm cells undergoing the process of spermatogenesis from an autoimmune attack [30]. However, if this barrier is broken, sperm antigens will come into contact with the immune system and lead to the formation of antisperm antibodies and eventually autoimmune infertility [31] .
In regard to the role of autoimmunity in infertility, studies have shown that antisperm antibodies penetrate the blood-testis-barrier, bind to spermatozoa, and reduce their fertilization capacity. These antibodies may also inhibit the acrosome reaction, activate the complement cascade system to lyse sperm cells, and interfere with the sperm’s ability to recognize particular binding sites on the zona pellucida [32]. Furthermore, they enter the cervical mucus and inhibit the ability of spermatozoa to penetrate it. Overall, the improper cellular or humoral immune response against sperm antigens causes dysfunction of spermatozoa and is a possible contributing factor to the unexplained infertility seen in males.
Defects in Genetic Integrity
Compromises in genetic integrity have been significantly correlated with UMI. Such compromises in DNA can take the form of insertion or deletion of bases, cross-linkage of strands, chromosomal anomalies, as well as single or double-stranded breaks [33]. Current research suggests that there is a causal relationship between defective spermatozoa DNA and male infertility [34]. This was demonstrated in a study that performed microarray analyses on spermatozoa mRNA, which illustrated differential expression of many genes between normozoospermic infertile men and fertile men. This indicates that males with unexplained infertility have distinct genome expression profiles specific only to UMI [35–37].
Overall, it is evident that large amounts of DNA damage in spermatozoa can interfere with a man’s ability to achieve a natural, viable pregnancy. Further research needs to be conducted on the effects of DNA damage on pregnancy outcomes as well as the threshold of damage that allows for normal functioning of spermatozoa [33].
Fertilization Defects
Fertilization of the oocyte is a complex and arduous process carried out by spermatozoa. It is a multi-step event beginning with (1) capacitation, (2) hyperactivation, (3) sperm-zona pellucida binding, (4) acrosome reaction, (5) penetration of the zona pellucida, (6) sperm-oocyte fusion, (7) cortical and zona reaction, and finally (8) post-fertilization events. Each component of this process must be carried out in a precise manner because interference with any of these steps can potentially lead to infertility [38]. Specifically, those with UMI have been shown to have reduced levels of protein phosphorylation, which is necessary for the process of capacitation [39]. Moreover, normozoospermic infertile men have demonstrated decreased hyperactivation of spermatozoa as well as defects in the proteins necessary for the zona pellucida induced-acrosome reaction [40–42]. Overall, a variety of anomalies can take place during the process of fertilization and contribute to unexplained infertility in males.
Oxidative Stress
Oxidative stress is defined as an imbalance between reactive oxygen species (ROS) and antioxidant defense mechanisms in the body [43]. ROS comprise a class of radical and nonradical oxygen derivatives [44]. Not only do ROS include oxygen radicals such as the hydroxyl radical, superoxide radical, and hydrogen peroxide but also a subclass of nitrogen-containing compounds collectively known as reactive nitrogen species (RNS). Examples of RNS include peroxynitrite anion, nitroxyl ion, nitrosyl-containing compounds, and nitric oxide [44, 45]. The most common ROS that is produced by spermatozoa is the superoxide anion radical; this is turn forms hydrogen peroxide (strong oxidizer) on its own or by the action of superoxide dismutase (SOD) .
Physiologic Role of Free Radical Species
Studies have shown that physiological levels of ROS are required for many baseline bodily functions [44]. Moreover, appropriate concentrations of ROS allow for proper signal transduction, mediation of cytotoxic events, and facilitation of inflammation via prevention of platelet aggregation and neutrophil adherence to endothelial cells [44]. These free radical species also serve as signaling molecules or second messengers, as well as aid in the production of hormones, regulation of tight junctions, and mediation of apoptosis. In regard to the male reproductive system, low ROS levels are necessary for capacitation, hyperactivation, acrosome reaction, zona pellucida binding and fertilization capacity of spermatozoa and promote normal semen parameters, such as sperm motility, morphology, and viability [19, 28, 46].
Detrimental Role of Reactive Oxygen Species
Although ROS are necessary for normal physiological functions, excess levels overwhelm the body’s natural antioxidant capacity . Due to the unpaired electron in their outer orbit, ROS are highly reactive and interact with a variety of lipids, proteins, and nucleic acids in the body. Such reactions are extremely harmful for reproductive potential and possibly contribute to testicular dysfunction, decreased gonadotropin secretion, and abnormal semen parameters [47]. While ROS affect a variety of reproductive functions, their reactive nature leads to the generation of more free radicals. This, in turn, perpetuates a chain of reactions creating tissue damage in the form of oxidative stress [45] .
In the male reproductive system, ROS are mainly produced by immature spermatozoa, macrophages, and polymorphonuclear leukocytes. Specifically, the latter two represent the majority of seminal leukocytes that generate ROS [48]. Conditions such as varicocele and leukocytospermia stimulate these leukocytes, among other inflammatory cells, to produce large amounts of ROS. Moreover, lifestyle habits such as smoking are strongly correlated with increased ROS production [49]. Additionally, these free radical species have been associated with cardiovascular disease due to the oxidation of low density lipoprotein (LDL) within the vascular endothelium. Oxidative stress also contributes to reperfusion injury following ischemia well as tissue injury after radiation therapy. Furthermore, infections such as Helicobacter pylori and neurodegenerative diseases such as Alzheimer’s and Huntington’s disease have been also been linked to the accumulation of oxidative stress [48] .
Role of Oxidative Stress in Unexplained Infertility
In addition to contributing to a variety of conditions , there is growing evidence that oxidative stress is involved in many aspects of UMI (Fig. 10.1). Studies have shown that when compared to fertile men, normozoospermic infertile males have elevated ROS levels measured by the malonaldehyde levels and protein carbonyl groups. [1, 50]. This is also evident by lower reactive oxygen species-total antioxidant capacity (ROS-TAC) scores in patients with UMI, which indicates elevated levels of seminal oxidative stress [51, 52]. Moreover, studies report high ROS dysfunction in UMI patients. This dysfunction can be in the form of reduced fertilization capacity, impairment of sperm metabolism, and lipid peroxidation of polyunsaturated fatty acids within the sperm plasma membrane [53] . Detrimental effects of high ROS on semen parameters such as motility, viability, morphology have been demonstrated by numerous studies [54, 55]. Not only does oxidative stress have negative consequences on sperm parameters but also on the DNA of sperm cells [50]. Studies have shown that patients with UMI have a significantly higher DNA fragmentation index ( > 30 %) induced by toxic levels of ROS when compared to those who are fertile [56]. Specifically, a fragmentation index > 30 % is associated with lower chances of achieving pregnancy by natural conception or by insemination [57]. This finding of increased ROS levels may indicate that seminal oxidative stress may be involved in the pathogenesis of sperm DNA damage in these patients [56]. It has also been suggested that specific genetic polymorphisms can promote the accumulation of oxidative stress within the seminal plasma, such as the glutathione S-transferase Mu-1 (GSTM1) gene polymorphism. Studies have reported that men with idiopathic and unexplained infertility who have the GSTM1 null genotype had significantly higher levels of seminal oxidative stress than those who possessed the GSTM1 gene. Therefore, in patients suffering from infertility, the GSTM1 polymorphism might be integral to determining the susceptibility of spermatozoa to oxidative damage [58]. Additionally, studies report that UMI patients with excess levels of ROS have numerous mutations on genetic analysis of sperm mitochondrial DNA. Examples of such alterations include nucleotide changes in the ATPase and nicotinamide adenine dinucleotide dehydrogenase genes. These DNA mutations may be the underlying etiology of a male’s unexplained infertility [59]. Furthermore, reactive oxygen species-induced DNA damage may accelerate the process of germ cell apoptosis, leading to the decline in sperm counts associated with male infertility [60]. Overall, it is clear that the oxidative stress may play a role in UMI via the mechanism of spermatozoa DNA damage .
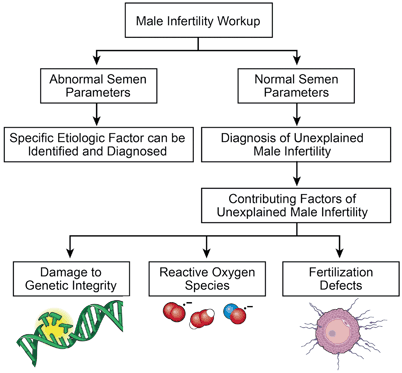

Fig. 10.1
Schematics of unexplained male infertility resulting in production of reactive oxygen species, genetic defects as well as fertilization defects
Measurement of Oxidative Stress
A number of methods have been utilized in the laboratory setting to measure ROS [45]. Direct methods include cytochrome c reduction, electron spin resonance, and nitroblue tetrazolium technique (NBT, and xyenol orange-based assay). Indirect methods of measurement include the Endtz test, redox potential (GSH/GSSG), measurement of lipid peroxidation levels, chemokines and measurement of DNA damage, and measurement of reactive nitrogen species by Greiss reaction and fluorescence spectroscopy [61] .
Assessment of ROS by Chemiluminescence
The chemiluminescence assay is one of the most commonly used techniques to measure seminal ROS levels in clinical andrology laboratories . The two commonly used probes are luminol (5-amino-2,3-dihydro-1,4-phthalazinedione and 3-aminophthalic hydrazide) and lucigenin ( N, N’-dimethyl-9,9’-biacridinium dinitrate). Both H2O2 and O2 ●− are involved in luminol-dependent chemiluminescence because both catalase and SOD can disrupt the luminol signal very efficiently. A luminescent signal is produced with luminol through a one-electron oxidative event mediated by hydrogen peroxide (H2O2) and either endogenous peroxidase or by addition of horse radish peroxidase. Superoxide anion (O2 ●−)is an essential intermediate for the luminol-dependent chemiluminescence. Also, the redox cycling activity associated with this probe allows the significant amplification of the signal and allows easy measurement of H2O2. There are a variety of luminometers available and these may be single tube or multiple tube luminometers [61] .
Luminol measures both intracellular and extracellular ROS such as hydrogen peroxide, and superoxide anion, on the other hand, lucigenin measures only extracellular ROS, and in particular superoxide anion.
For the actual assay, luminol (5 millimolar) is used to measure ROS in a clinical andrology lab setting. Chemiluminescence is measured using an instrument called luminometer (Fig. 10.2). Luminol is sensitive to light and the assay is performed in indirect light or in the dark. The samples are run in duplicate or in triplicate with appropriate negative and positive controls. Test samples are comprised of 400 µL of completely liquefied seminal ejaculate + 10 µL luminol; positive control: 400 µL of phosphate buffered saline (PBS) and 50 μL hydrogen peroxide (30 %) + 10 μL luminol; and negative control: 400 μL PBS and 10 μL luminol. (Fig. 10.3) The measurement is for 15 min. The results are expressed as relative light units (RLU)/s/106 sperm. ROS levels > 20(RLU)/s/106 sperm are considered as positive.

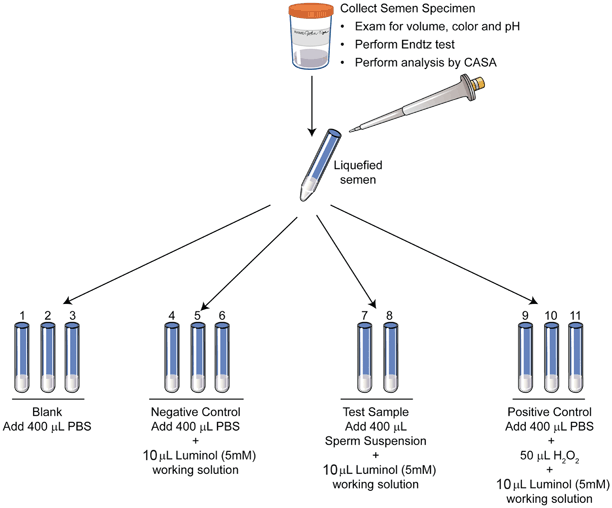

Fig. 10.2
A multitube Autolumat 953 plus luminometer used in the measurement of ROS by chemiluminescence assay. Multiple tubes can be loaded simultaneously for measuring ROS. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2011–2013. All rights reserved)

Fig. 10.3
Schematics of sample preparation for the ROS measurement. A total of 11 tubes are labeled from S1–S12: blank, negative control, patient sample, and positive control. Luminol is added only to all tubes except blank. Hydrogen peroxide is added only to the positive control
Flow Cytometry
Flow cytometry is a laboratory method used for analyzing the expression of cell surface markers and intracellular molecules. Oxidation of 2, 7 dichlorofluorescein diacetate (DCFH-DA) by ROS, which is generated within the cell, makes them highly fluorescent and can be used to measure formation of intracellular levels of hydrogen peroxide. Hydroethidine (HE) is another fluorescent probe that can be used for the measurement of intracellular levels of superoxide [61, 62].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





